Manuscript accepted on :April 04, 2008
Published online on: --
Plagiarism Check: Yes
Kishu Tripathi¹ and Shobha Kulshreshtha²
¹Surya College of Pharmacy, Lucknow.
²Head, Department of Pharmacology, SN Medical College, Agra.
Abstract
Metal ions after forming complexes with an antibiotics alters the antimicrorial activity of an antibiotics alone.
Keywords
Metal ions ; antibiotics
Download this article as:| Copy the following to cite this article: Tripathi K , Kulshreshtha S. Metalloantibiotics- II. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Tripathi K , Kulshreshtha S. Metalloantibiotics-I. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=351 |
Antitubercular Compounds
Isoniazid
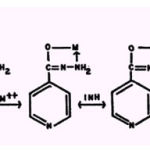
Molecular structure and affinity for metallic cations. Isoniazid (isonicotinic acid hydrazide;INH) chelates metallic ions to form 1:1 and 2:1 INH-metal complexes:
Fallab and Erlenmeyer (35) observed 2:1 isoniazid-Cu2+ complexes by spectrophotometric methods, and Albert (4) reported that in alkaline solutions, Cu2+, Ni2+, Co++, and Zn2+ form soluble, and Fe2+ and Mn2+ form insoluble,1 :1 isoniazid-metal complexes.
 |
Scheme 1 |
 |
Scheme 2 |
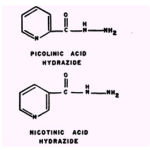
Picolinic acid hydrazide is a much better chelating agent than INH but is only one-eighth as effective against Mycobacterium tuberculosis; and nicotinic acid hydrazide is as good a chelating agent as INH but has no antitubercular activity.
In contrast to observations cations suppress the action of INH, the ability of the drug to inhibit respiration of M. tuberculosis is enhanced by Cu2+ (82) and the development of resistance by bacteria to INH is retarded if excessCu2+is present in the environment (88).Moreover, the Cu2+ complex of INH is a much stronger inhibitor of mammalian or microbial catalase than is the free drug (66A). Thus, Maher et al. (66A) have proposed that INH might owe its antitubercular activity to the formation of a Cu2+complex that can successfully compete with peroxide for sites on the catalase molecules of the tubercle bacilli.In our laboratory, the toxicity of Ni2+, Co2+, Zn2+, and Cd2+ towards the growth of species of Bacillus and Pseudomonas was observed to be suppressed by isoniazid.
Streptomycin
The streptomycin molecule possesses numerous centers for chelation and prepared streptomycin chelates ofCo++ Cu2+, Ni2+, and Co++. Of the three complexes,only the Cu2+ chelate is free of contamination by metal hydroxide; this complex was found to have a 1:3 streptomycin-Cull molar ratio. Several potential sites exist on the streptidine portion of the molecule:
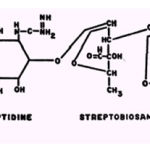 |
Scheme 3 |
The Cu++ chelate prepared by Foye et al. (40) possesses approximately thirty-seven per cent of the toxicity of the antibacterial potency of pure streptomycin; Co++ chelate, nine per cent; and the Ni++ chelate, less than six percent.Previously Donovick et al. (32) had demonstrated that the inhibition of growth of Klebsiella pneumoniae by streptomycin is suppressed strongly by Ca2+, Mg++, and Ba++, but not by Na+, K+, Al3+, or NH4+. Unfortunately, such multivalent ions as Cu++, Ni++, Co++, Zn++, Mn2+, Fe+(+), and Al3+ were not included in the tests with K. pneumoniae.Inhibition of growth of Micrococcum pyogenes by streptomycin has been reported to be very slightly enhanced by Co2+, (104). Inhibition of respiration of Escherichia coli by the drug (16) and uptake of streptomycin by algal cells (83) are suppressed by Ca2+ and Mg2+but not by monovalent cations.
References
- Albert, A. 1953 The Affinity Of Isonicotinic Hydrazide For Metals. Experientia,9,
- Berti, T. 1950 Fenomeni Di Interferenza Tra Elettroliti E I. Streptomicina. Arch. Internat. Pharmacodynamie, Adsorption Studies Of Aureomycin Hydro-82, 23-31.
- Donovick, , Bayan, A. P., Canales, P.,And Pansy, F. 1948 Differential Effects Of Various Electrolytes On The Action Of Streptomycin. J. Bacteriol., 56, 125-137.
- Feeney, E. 1951 The Antagonistic Activities Of Conalbumin And 8-Hydroxyquinoline (Oxine). Arch. Biochem., 34,196-208.
- Foye, O., Lange, W. E., Swintosky, J. V., Chamberlain, R. E., And Guarini, J. R. 1955 Metal Chelates Of Strepto- Mycin. J. Am. Pharm. Assoc., 44, 261-263.
- Maher, It., Speyer, J. F., And Levine, M. 1957 Mode Of Action Of Isoniazid. 68, Amer. Rev. Tuber., In Press.
- Pothman, P. J., And Sttttgen, G. 1955 Iler Einfluss Von Isonicotinsauerehydracid Und Seinem Kupfercomplex Auf Atmung Und Wachstum Von Z. Hyg. Infectionskrankh., 141, 359-362.
- Pramer, 1956 Absorption Of Antibiotics By Plant Cells. Ii. Streptomycin. Arch. Biochem. And Biophys., 62, 265-273.
- Roth, W., Prijs, , And Erlenmeyer, H. 1954 Metallionen Und Resistenz-Bildung Bei Tbe-Kulturen Ii. Helv. Chim. Acta, 37, 2010-2013.
- Trace, J. C., And Edds, T. 1955 The Influence Of Cobalt On The Action Of Antibiotics. Am. J. Veterinarv Research, 15, 639-642.







