Manuscript accepted on :April 04, 2008
Published online on: --
Plagiarism Check: Yes
R. K. Mohamed Mutahar¹*, R. Ramesh1 , C. Nagesh2, and Shaista Omer3
1P.G. Department of Pharmaceutics, Faculty of Pharmacy, Dr.H.L.T. College of Pharmacy, Kengal, Channapatna, Bangalore (Rural)
2Department of Pharmaceutics, S. C. S. College of Pharmacy, Harapanahalli.
3Soniya College of Pharmacy. Dharward.
Abstract
The literature and market survey reveal that so far there is no preparation of niacin in the form of effervescent tablets (ET) and it is still available in the market as only a conventional tablet. Even this conventional tablet is prepared hardly by a handful of pharmaceuticals. The present study was aimed to formulate the niacin in the form of ET dosage form as an alternative to the available marketed few conventional tablets. ET was made up of effervescent granules (EG). EG are prepared by wet granulation method with non-reactive liquids using PVP as a binder with concentration ratios of 1: 2: 4. As the concentration of PVP is increased, the dissolution profile of niacin was decreased. The formulation F8 containing citric acid, tartaric acid and sodium bicarbonate in the ratio of 1:1:1 was considered to be the best formulation. All the prepared EG and ET are evaluated for the official tests and found to be within limits. In- vitro dissolution studies of formulation F8 shows good release by about 95.2% in 3.30 hours. All prepared formulations are slightly acidic (pH 4.0 to 4.2) to augment the taste of the solution. IR Spectra of formulation F8 shows, there is no interaction between niacin and excipients used. And the formulation F8 was most stable at temperature 25oC to 40oC. It conclude that, niacin can prepared in the form of “pleasantly flavored effervescent drink” of ET by compressing EG, which is prepared by wet granulation method with non-reactive liquids.
Keywords
Niacin; effervescent tablet; polyvinylpyrrolidine; sodium bicarbonate; dyslipidemia
Download this article as:| Copy the following to cite this article: Mutahar R. K. M, Ramesh R , Nagesh C , Omer S . Formulation, Development and in Vitro Evaluation of Effervescent Tablets of Niacin for Dyslipidemia. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Mutahar R. K. M, Ramesh R , Nagesh C , Omer S . Formulation, Development and in Vitro Evaluation of Effervescent Tablets of Niacin for Dyslipidemia. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=244 |
Introduction
The purpose of this study is to explore the possibility in the formulation of novel, flavored effervescent drink of niacin in the form of effervescent tablets (ET) for Dyslipidemia.
The formulation and evaluation of ET of niacin was taken up with the following aim: As is common with most of the dosage forms, niacin in the form of conventional tablet is associated with not only gastric irritation, but also poor absorption. To reduce this gastric irritation and to enhance absorption of drug, niacin can be given in the form of ET and to provide a suitable dosage form as an alternative to the available marketed few conventional tablets of niacin. Efficiently administering medicine is of utmost importance in order to obtain good therapeutic results. The most common way to give patients their medicine is by oral administration, through pills, tablets, capsules and syrups,1 that have some drawbacks. However, especially concerning pills, tablets and capsules, for example, all three take time to dissolve in the stomach and release the active ingredient. This increases the time that is needed to transport the drug from the intestine into the bloodstream. Dissolving the pill, tablet or contents of the capsule in water prior to ingestion is a common way to avoid this extra step in the stomach. Yet, the obtained solution is seldom clear and contains un-dissolved material, which remains unused in the dispensed containers, thereby assuring cent percent drug has not been given to the patient.
One pill alternative is the formulation of active ingredients as ET. When dissolved in water, the basic excipient (a carbonate) and the acid excipient (an organic acid) will react with each other, liberating carbon dioxide. Due to the dynamics of this process, turbulence is created and the active ingredient will dissolve more rapidly1 in the water, without any un-dissolved material in the solution. Thereby, assuring cent percent drug transportation into the blood stream.
Since, niacin is so far known to be a best example for drug that is sparingly soluble in water; effervescence is the method of choice in formulating active ingredients (niacin) with poor water solubility.
Niacin was first reported to affect lipids in 1955 2,is one of the oldest drugs used to treat Dyslipedemia and was most versatile in that favorably affect virtually all lipids parameters. Niacin was the best agent available for increasing HDL-C (increments of 30% to 40%); it also lowers triglycerides 35% to 45% (as effectively as fibrates and the more potent statins) and reduces LDL-C levels by 20% to 30%. Niacin is also the only lipid-lowering drug that reduces Lp(a) levels significantly, by about 40%. The pharmacological doses of regular (crystalline) niacin (>1 g per day) used to treat Dyslipedemia are almost completely absorbed, and peak plasma concentration (upto 0.24mM) are achieved within 30 to 60 minutes.3
The National Pharmacy Cardiovascular Council (NPCC) recommends niacin as first-line therapy for patients with hypertriglyceridemia (without diabetes) and for patients with isolated low HDL-C. Furthermore, the NPCC recognizes that niacin’s favorable effects on the overall lipid profile make it a valuable treatment option for patients with atherogenic or mixed Dyslipidemia, a condition characterized by elevated LDL-C and triglycerides.4
Despite its salutary effects niacin has its own side effects also. Historically, the use of niacin has been limited by cutaneous flushing, which is a prostaglandin D2-mediated vasodilation characterized by sudden warmth, redness, itching, and/or tingling on the face and truncal regions and is usually evident one to two hours after taking a dose of niacin.5 Flushing can be reduced by giving aspirin and taking the medication with meals.3
Material and Methods
Materials
USP Niacin RS, (Reference Standard) (Dry the Niacin at 105oC for 1 hour before using),tartaric acid, citric acid (anhydrous), sodium carbonate, sodium bicarbonate, polyvinylpyrrolidine (PVP), aspartame, sodium saccharine, talc, sod benzoate, polyethylene glycol 6000, magnesium stearate, sodium chloride, colors (Sunset Yellow), flavors, (orange booster) and all other chemicals AR grade, provided by Jagdale scientific research foundation, Bangalore.
Methods
Processing of effervescent granules (EG) and effervescent tablet (ET)
EG and ETrequires special environmental conditions: low relative humidity (RH) and moderate -to- cool temperatures in the processing areas are essential to prevent the granulations or tablets from sticking to machinery and from picking up moisture from the air, which may lead to tablet instability. In the present processing a maximum of 25% RH at a controlled room temperature (RT) of 250C or less, was found to be satisfactory to avoid the problem due to atmospheric moisture 6.
Preparation of effervescent granules (EG)
EG were prepared by wet granulation method with nonreactive liquids. First sieve all the ingredients with a # 60 sieve. Weigh and collect the required quantity of sieved ingredients (Table 1), (except flavor and the lubricants.) and weigh the clinically relevant dosage strength of 500 mg niacin. Both were placed in a tray and mixed with liquid binder, such as PVP (5% in anhydrous ethanol) to make a coherent mass. This mass was immediately passed through sieve no. 16, which was superimposed sieve no. 44, then spread out on a tray, and dried in a oven at 500C for 1 hour. These EG were immediately transferred to suitable dessicator, and tightly closed6.
Preparation of effervescent tablets
Blend the thus obtained EG thoroughly with the weighed and sieved flavor and lubricants (Table 1). Now the ET can be prepared by compressing the lubricated EG on single punch tablet machine at a RH of 25% and RT of 250C, using 13 mm in diameter and flat faced, beveled edge die and punch set, ET weighing 3.165g each. pass through curing oven; cool; and package in aluminum foil 6.
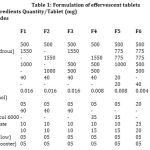 |
Table 1: Formulation of effervescent tablets |
Evaluation of effervescent granules







