Manuscript accepted on :April 04, 2008
Published online on: --
Plagiarism Check: Yes
Jagadeesh Panda¹ , P. Venkat Rao¹*, S. Mukunda Valli², T. Prabhakar³, S. Suneetha³
¹Raghu College of Pharmacy, Dakamarri, Visakhapatnam.
²Yalamarty Pharmacy College , Tharluwada ,Visakhapatnam.
³A.U.College of Pharmaceutical Sciences, Andhra University, Visakhapatnam.
Abstract
Paliperidone is a psychotropic agent belonging to the chemical class of benzisoxazole derivatives. Chemically, it is the major metabolite of the widely used atypical antipsychotic drug, resperidone. Paliperidone is indicated for acute and maintenance treatment of schizophrenia. Paliperidone-ER (sold as INVEGA) is the first oral atypical antipsychotic with an extended release, which is achieved by osmotic-controlled release oral delivery system. Paliperidone is a centrally active dopamine Type 2 (D2) antagonist which is metabolized by dealkylation, hydroxylation, dehydrogenation and benzisoxazole scission.Paliperidone gradually rise to reach peak plasma concentration (Cmax ) approximately 24 hours after dosing. The maximum pharmacokinetics of paliperidone administration are dose-proportional within the recommended clinical dose range (3 to 12 mg).The terminal elimination half-life of paliperidone is approximately 23 hours
Keywords
Paliperidone; risperidone; antipsychotic; schizophrenia
Download this article as:| Copy the following to cite this article: Panda J , Rao P. V , Valli S. M, Prabhakar T , Suneetha S. Clinical and Pharmacological Review on Novel Atypical Antipsychotic Drug: Paliperidone. Biomed Pharmacol J 2008;1(1). |
| Copy the following to cite this URL: Panda J , Rao P. V , Valli S. M, Prabhakar T , Suneetha S. Clinical and Pharmacological Review on Novel Atypical Antipsychotic Drug: Paliperidone. Biomed Pharmacol J 2008;1(1). Available from: http://biomedpharmajournal.org/?p=292 |
Introduction
Paliperidone, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidine-4-one is an atypical antipsychotic which is indicated in the treatment of schizophrenia as well as manic and mixed episodes occurring in conjuction with Bipolar I Disorder and depression1-2. Paliperidone is a risperidone with an extra hydroxyl group i.e, 9-hydroxyrisperidone. Paliperidone-extended release (ER) is achieved by the osmotic-controlled release (OROS), a well established technology already in use for CNS drugs1. Paliperidone exhibits powerful efficacy for many patients experiencing symptoms of schizophrenia including those who may benefit from a change in therapy.
Chemical Structure
Systematic (IUPAC) name7
3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidine-4-one
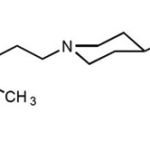 |
Scheme 1 |
Molecular Formula
C23H27FN4O3
Molecular Mass
426.49
Physical Properties
Solubility
Paliperidone is a white to yellow non-hygroscopic powder. It is sparingly soluble in 0.1N HCl and methylene chloride. It is insoluble in water, 0.1N Sodium Hydroxide and hexane; and slightly soluble in N,N-dimethylformamide.
Stability
Paliperidone remains stable at normal conditions of temperature and pressure.
Identification
The identification of paliperidone includes tests for appearance (visual examination), identification by IR (Ph. Eur) and HPLC, heavy metals (USP), residue on ignition and sulphated ash (USP ), water content by (Karl-Fischer), assay (HPLC), related substances (HPLC), residual solvents (GC) and particle size (Laser diffraction)2.
Dosage Forms And Strengths1
INVEGA® Extended-Release Tablets are available in 3 mg (white), 6 mg (beige), and 9 mg (pink). All tablets are capsule shaped and are imprinted with either “PALI 3”, “PALI 6”, or “PALI 9”.
Dose And Administration
The recommended dose of paliperidone Extended-Release Tablets (INVEGA) is 6 mg once daily, administered in the morning. Initial dose titration is not required. Some patients may benefit from higher doses; up to 12 mg/day, and for some patients, a lower dose of 3 mg/day may be sufficient. When dose increases are indicated, small increments of 3 mg/day are recommended. The maximum recommended dose is 12 mg/day1.
Paliperidone ER tablet can be taken with or without food. It must be swallowed whole with the aid of liquids. Tablets should not be chewed, divided, or crushed.
Pharmacology
Mechanism of action
The mechanism of action of paliperidone, as with other drugs having efficacy in schizophrenia, is unknown, but it has been proposed that the drug’s therapeutic activity in schizophrenia is mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism.
Pharmacodynamics
Primary pharmacodynamics
Paliperidone is a centrally active dopamine Type 2 (D2) antagonist and with predominant serotonin Type 2 (5HT2A) activity. Paliperidone is also active as an antagonist at α1 and α2 adrenergic receptors and H1 histaminergic receptors, which may explain some of the other effects of the drug. Paliperidone has no affinity for cholinergic muscarinic or β1– and β2-adrenergic receptors3.
The binding profiles for paliperidone, its enantiomers and risperidone are comparable. Several in vivo studies were performed in rats and dogs. In rats, paliperidone was slightly less potent than risperidone at early time intervals, but became equipotent at later time intervals, probably reflecting a slower rate of brain penetration. In dogs, paliperidone, its enantiomers and risperidone were roughly equipotent against apomorphine-induced emesis2.
Secondary pharmacodynamics
Dopamine secreted in the portal hypophyseal circulation inhibits prolactin release. By antagonizing this tonic inhibitory action of endogenous dopamine, D2 receptor antagonists elevate prolactin release. Anti-adrenergic and anti-histaminergic effects are suspect to elicit hypotensive and sedative effects. Hyperprolactinemia is expected due to the D2-receptor antagonism.
Pharmacokinetics
Paliperidone is presented as a prolonged-release formulation. The goal of the development program was to identify an extended-release formulation that would enhance the initial tolerability and permit initiation of treatment at an effective dose without the need for initial dose titration. Invega prolonged release tablets are based on the patented OROS® (Oral Osmotic System) Push-PullTM technology delivery system, designed to deliver the paliperidone active substance in a controlled manner over 24 hours, thereby achieving an effective once-a-day treatment for schizophrenia10.
Following a single dose, the plasma concentrations of paliperidone gradually rise to reach peak plasma concentration (Cmax ) approximately 24 hours after dosing. The maximum pharmacokinetics of paliperidone administration are dose-proportional within
(Visited 456 times, 1 visits today)






