Manuscript accepted on :23-Mar-2019
Published online on: 10-04-2019
Plagiarism Check: Yes
Reviewed by: Sharad Kamble
Second Review by: Abdullah Noohu
Yakaiah Vangoori1, Anusha Dakshinamoorthi2 and S. Kavimani3
1Pharmacology. Santhiram Medical College, Nandyal (AP). PhD Scholar- Sri Ramachandra Institute of Higher Education and Research (SRIHER) – Chennai, India.
2Pharmacology, Sriramachandra Institute of Higher Education and Research. Chennai, India.
3Pharmacology- Mother Theresa Post Graduate and Research Institute of Health Sciences. Pondicherry, India.
Corresponding Author E-mail: drdanusha@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1688
Abstract
The effect of the ethanolic extract of Myristica fragrans was evaluated on cafeteria diet induced body weight, glucose and lipid elevations in albino rats. 30 rats were taken randomly and divided into five groups and six each. Group-1 normal control and Group 2-5 were give cafeteria diet for 6 weeks to induce obesity and treatment period was 10 weeks. After 70 days of treatment, the extract, at doses of 200 and 400mg/kg, significantly reduced the body weight, glucose and lipid levels (p < 0.001) dose dependently. The standard drug Orlistat at 50mg/kg effectively prevented the body weight, glucose and lipid levels when compared with control and test groups. With these observations and previous data, the study concludes that Myristica fragrans extract can stimulate AMP-Kinase enzyme system and can reduce glucose and lipid concentrations. This may be useful for obesity treatment.
Keywords
Glucose; LFT; Mace; Myristica Fragrans; Obese; Orlistat; Pancreatic Lipase; RFT; Weight Gain
Download this article as:| Copy the following to cite this article: Vangoori Y, Dakshinamoorthi A, Kavimani S. Effect of Myristica Fragrans Extract on Lipid Profile, Glucose, Body Weight, Food Intake, Liver and Renal Functions in Experimental Obese Rats. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Vangoori Y, Dakshinamoorthi A, Kavimani S. Effect of Myristica Fragrans Extract on Lipid Profile, Glucose, Body Weight, Food Intake, Liver and Renal Functions in Experimental Obese Rats. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2OZGLpr |
Introduction
As per previous research reports, hyperlipidemia can cause cardiovascular problems, hyperglycemia, obesity and have major role in pathogenesis of various tissues. Obesity can induce insulin resistance, there by hyperglycemia, increase in blood pressure, dyslipidemia, collectively called “metabolic syndrome”.1 Obesity, which has been termed as “New World syndrome” is now considered by world health organisation as a global problem. According to 2014-WHO report, 1.9 billion adults are overweight, of which 600 million are obese. The obesity is spreading to not only developed countries, but also all over the world 4.5 million people were died in 2013 due to overweight and obesity.2 Obesity can be treated by reducing lipid levels. Animal models are useful tools for obesity research as they readily gain weight in short period when fed with high-fat diet. Human obesity clinical features are almost similar to animal models of obesity physiologically.3 Therefore diet induced hyperlipidemia model was selected to observe the hypolipidemic effect of Myristica fragrans extract in the present study. Plants contain different chemical compounds and can act on specific cell sites. Many new drugs have been produced from the plant source, and these find use among the most common complementary and alternative medicine systems.4 Presence of multiple-Phytochemical combinations in plant drugs may result in synergistic effect by their action on multiple molecular targets, thus offering advantages over treatments which use a single constituent.5 The development of standardized, safe and effective drugs from plant origin can provide economical alternatives for the treatment of obesity. Therefore, there is a need to develop and screen large number of plant extracts and this approach can surely be a driving force for the discovery of anti-obesity drugs from medicinal plants. Myristica fragrans is an aromatic evergreen tree cultivated in South Africa, India, and other tropical countries. It is commonly used spice. It has been prescribed in Asia for many diseases like rheumatism, muscle spasm, to decrease appetite, and Hyperlipidemia.6,7 It was found hypolipidemic effects in rabbits in some previous research reports8,9 but there are no anti-obesity studies with Myristica fragrans. It has been reported that the spice can be toxic when ingested in large quantities (1-3 nutmegs) causing convulsions, hallucinations, and possibly death.10 Some active compounds present in Nutmeg can also alter physiological functions of hepatic and renal systems. Hence, the objectives of the current study are to investigate the hypolipidemic and Antiobesity effect of MF in high fat-fed rats and to evaluate the pharmacological activities on hepatic and renal functions.11
AIM
To evaluate the effect of ethanolic extract of Myristica fragrans on body weight, lipid profile, hepatic and renal systems in experimentally induced obese wistar albino rats.
Material and Methods
Fresh and dried Mace was purchased from wholesale grocery store for the preparation of extract. Authentication was done by Dr.K.Venkata Ratnam. M.Phil., PhD, Assistant Professor of Botany, Rayalaseema University. Kurnool. A.P. Dried mace was ground to a fine powder and extract was prepared by using Soxhlet apparatus with ethyl alcohol as solvent.12
Research Design
Thirty (30) healthy albino rats weighing between 150-180 gm were taken from central animal house, Santhiram Medical College. The rats were housed under 22±2°C temperature, 40-60% humidity and 12-12±1 h light-dark cycle and allowed food and water ad libitum. Rats were randomly divided in to five groups of six rats each (n= 6). To induce obesity, rats were fed on high fat diet (HFD/CD) for 6 weeks and to test the plant extract efficacy, rats were administered with MFE along with CD for 70 days. Before starting the study, Institutional Animal Ethics Committee (IAEC) permission was taken (IAEC/SRMC/2017/2). For all the rats, body weight, Food intake, normal lipid profile, Glucose levels was done before starting the study.
Induction of Obesity
Normal control rats (Group-I) were fed with standard pellet diet of standard composition containing all the recommended macro and micronutrients prepared according to AIN-93 guidelines with water ad libitum. Group-II to Group-V rats were initially fed with cafeteria diet (CD) for 6 weeks to induce obesity (20 g daily) and from 7th week on wards, different doses of Myristica fragrans (200, 400 mg/kg b.wt) were supplemented for 70 days (10weeks) along with CD as mentioned. Total duration was 16 weeks.
Hyper calorie/cafeteria diet (CD): (It consisted of 3 variants) (13)
Condensed milk + bread + peanuts + pellet chow (4:1:4:1),
Chocolate + biscuits + dried coconut + pellet chow (3:2:4:1), and
Cheese + boiled potatoes + pellet chow (4:2:1).
The different variants were fed on alternate days throughout the treatment period (70days)
Experimental design
Group 1: Standard pellet diet- Normal control
Group 2: Cafeteria diet (CD)-Obese control
Group 3: Obese + Orlistat 50 mg/kg
Group 4: Obese + MFE 200 mg/kg
Group 5: Obese + MFE 400 mg/kg
Food Intake
All the rats were fed with normal laboratory Pellet diet. Food was presented in the form of pellets in grams. FI was measured manually. A known amount of food was given to the animal. After 24 hours, the leftover food was weighed again and the amount consumed was calculated and then for every 10 days up to 70 days.
Consumption of food = Total quantity of food given to rat – leftover food
At the end of the study period, body weight, lipid profile-TC (Total cholesterol), TG (Triglycerides), LDL (Low density lipoprotein), VLDL (Very low density lipoprotein), HDL (High density lipoprotein), liver function test-SGPT (Serum glutamic puruvic transaminases), SGOT (Serum glutamic oxaloacetic transaminase), ALP (Alkaline phosphatase) and renal function test (CREATININE, UREA, URIC ACID) was done to examine MFE effects on body weight and lipid profile.
Statistical Analysis
All the data was presented as mean ± SEM. The one way ANOVA was used to analyze the data, followed by Dunnett’s test. The results were measured statistically using SPSS Statistics 20.0 (IBM software) for the analysis. The results considered significant if p values < 0.05.
Results and Discussion
At the end of 70th day of treatment, Body weight was recorded and blood was withdrawn from retro-orbital sinus from all the groups and assessed for lipid profile and compared with control group. When plasma lipid levels were analyzed, CD (Cafeteria Diet) or high fat diet caused substantial elevation in TC, TD, LDL, VLDL, and reduced the levels of HDL when compared with normal control group (Group-1). Treatment with ethanolic extract of Myristica fragrans significantly (p<0.05) and dose dependently reduced the concentrations of TC, TD, LDL, VLDL, but increased the levels of HDL when compared to CD fed obese control rats (Group-2) depicted in table 1.
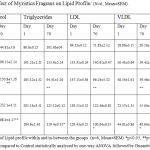 |
Table 1: Effect of Myristica Fragrans on Lipid Profile: (N=6, Mean±SEM).
|
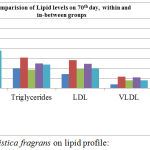 |
Figure 1: Effect of Myristica fragrans on lipid profile:
|
MF-Extract Effect on B
(Visited 861 times, 1 visits today)






