Sangeeta Mohanty*, Sthitapragnya Panda, Aslesha Bhanja, Abhisek Pal and Si Sudam Chandra
School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, India.
Corresponding Author E-mail: sangeetamohanty@soauniversity.ac.in
DOI : https://dx.doi.org/10.13005/bpj/1624
Abstract
Recent advances in science and technology radically changed the way we detect, treat and prevent different diseases in all aspects of human life. Rheumatoid arthritis (RA) is a chronic, systemic, progressive, autoimmune disease in which the body’s immune system whose major role is to protect the health by attacking foreign bacteria and viruses are mistakenly, attacking the joints resulting in thickened synovium, pannus formation, & destruction of bone, cartilage. Still now researchers are unable to know the exact cause of this disease. However, it is believed that genes and environmental factors play a role in development of RA. In this review, we discuss the Pathophysiology, predictors, & factors involved in pathogenesis of RA. We also discuss the Conventional therapeutic agents for Rheumatoid Arthritis. More importantly, we extensively discuss the emerging novel drug delivery systems (NDDS) like nanoparticles, dendrimers, micelles, microspheres, liposomes, and so on as these are the promising tools having successful applications in overcoming the limitations associated with conventional drug delivery systems. Although several NDDS have been used for various purposes, liposomes have been focused on due to its potential applications in RA diagnosis and therapy. In addition, we discuss the therapeutic effectiveness and challenges for RA by using these novel drug delivery systems. Finally, we conclude by discussing the future perspectives.
Keywords
Disease Modifying Anti-arthritic Drugs; Glucocorticoids; Liposome; NSAIDs; Rheumatoid Arthritis
Download this article as:| Copy the following to cite this article: Mohanty S, Panda S, Aslesha Bhanja A, Pal A, Chandra S. S. Novel Drug Delivery Systems for Rheumatoid Arthritis: An Approach to Better Patient Compliance. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Mohanty S, Panda S, Aslesha Bhanja A, Pal A, Chandra S. S. Novel Drug Delivery Systems for Rheumatoid Arthritis: An Approach to Better Patient Compliance. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2UtjEoX |
Introduction
RA is a systemic disorder characterized by inflammation of joint .It is an autoimmune disease that shows chronic systematic inflammatory symptoms due to tissue abnormality, localized damage in different parts of cartilage, bone, tendon and ligament. Inflammatory cytokines causes activation of the macrophages which leads to swelling of joints, damage to cartilage, erosion of bone, functional impairment and stiffness. Several important drugs are been used for RA treatment like Glucocorticoids, DMARDS, NSAIDS, and biological drugs. However, due to low bioavailability and high clearance rates, frequent dosing is been administered to improve the therapeutic effects. These treatments further increases the risk of occurring unwanted side effects like infection, tumors and GI toxicity. However, it is known that patients suffering from RA show symptoms such as acute cardiovascular events, myocardial infarction, shown in Fig 1. Around 40/100,000 of the general population is suffering from this disease worldwide. Out of which percentage of women suffering from RA is 3.6% in contrast to men having 1.7%. Although, it can affect several other joints in the body but the main affected area is observed in wrist joint.1 Symptoms like tenderness seen in large joints whereas swelling occurs in small joints .Different predicators of Rheumatoid arthritis are represented in Fig 2.
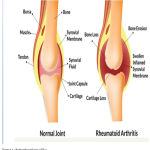 |
Figure 1: Pathophysiology of RA.
|
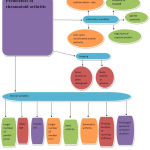 |
Figure 2: Predicators of RA.
|
Rheumatoid Arthritis Pathogenesis
Rheumatoid arthritis is characterized by the infiltration of inflammatory cells into the joints .The pathogenesis of RA consist of a complex process involving Pannus formation ,synovial fibroblast proliferation causing infiltration of T-cells, B-cells ,macrophages and plasma cells .However, it consist of mediators to form a network of interdependent system including cytokines, tumor necrosis factor, interleukins which stimulates the development of pro-inflammatory response on the cell .Hence, aim of treatment is to prevent joint destruction by improving functional ability ,decreasing pain & inflammation in order to maintain a normal lifestyle.
Currently available treatment for rheumatoid arthritis includes several NSAIDs, Glucocorticoids, DMARDs, biological anti-rheumatic drugs. Although, NSAIDs are having analgesic and anti-inflammatory properties but it does not prevent joint destruction whereas Glucocorticoids are used to reduce the progression of joint damage.2 The DMARD /Biological anti-rheumatic drugs are the major groups of the drug that are been used in treatment of RA which shows the potential to prevent from joint damage. Three major factors involved in the pathogenesis of rheumatoid arthritis are classified below and is summarized in Fig 3 & 4.
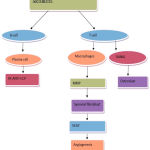 |
Figure 3: Pathogenesis of RA.
|
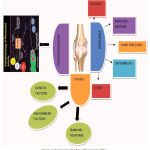 |
Figure 4: Factors involved in pathogenesis of RA.
|
Preclinical RA
Preclinical RA occurs with increase level of diseased condition in association with the presence of auto-antibodies in the body .Most specifically, it includes Calpastatin, p68, RA33, peri nuclear factor and IgM rheumatoid factor. According to American Rheumatoid Association, diagnosis of RA occurs due to the presence of serological features (RF).
Genetic Aspects of RA
The genetic aspects of RA is mainly determined by meta-analysis and genome-wide association studies (GWASs). Gene therapy of rheumatoid arthritis is capable to transfer genes frequently and efficiently to various joints without any destruction. Several proteins that encodes with genes includes Protein tyrosine phosphate non receptor 22(PTPN22), TNF -induced protein 3(TNFAIP3), Cytotoxic T-Lymphocyte antigen -4(CTL44), C-C chemokine receptor type 6(CCR6) with activator of 4(STAT4).3
Environmental factors of RA
Neither only genetic factors nor environmental factors are solely responsible, rather bacterial and viral infections are also responsible for the etiogenesis of RA. Bacterial and viral infection consists of mechanisms including presence of molecular mimicry, epitope spreading and expression of the super antigen. From B-cells, the citrulline – specific pathogenic T-cell and antibodies of anti-citrullinated protein (ACPAs) are activated .Furthermore, ACPAs reaction with citrullinated proteins, helps to induce local inflammation causing chronic rheumatoid arthritis .Along with these above mentioned factors, different environment risk factors such as smoking, alcohol, breast feeding, birth weight and socioeconomic conditions also plays important role in the pathogenesis of RA.4
Therapeutic Approaches (Conventional Drug Delivery Systems)
Till now, no permanent cure has been developed for RA, but by various therapeutic approaches only relieve can be given to patients from the discomfort & pain cause by inflamed arthritis, reducing the joint damage, deformities and thus to provide a healthy active life. RA therapeutics are broadly classified into four different classes depending on the articular function, articular damage and on the synovial inflammation degree.5 Current conventional therapeutics for RA patients include NSAIDs, DMARDs, Glucocorticoids, and Biological drugs.Conventional treatment strategies for rheumatoid arthritis has been summarized in Table 1. During RA treatment, it has been observed that NSAIDs, DMARDs and modern biologics shows progressive results .6 several current novel drug delivery systems for RA are represented in Fig 5.
Table 1: Conventional therapeutic agents for Rheumatoid Arthritis.
| Drug Classification | Instance | Mechanism of action | Mean size | Delivery strategy | Side effect |
| DMARDs | Methotrexate | Immunosuppresion ,inhibition of genetic material synthesis | 100 | EPR | Gastrointestinal reaction, liver and kidney failure |
| Glucocorticoids | Prednisolone,
Dexamethasone |
Impact on the levels of inflammatory cytokines and immunosuppresion | 90-110,
100 |
Hypertension, atherosclerosis, osteoporosis, osteonecrosis | |
| NSAIDs | Indomethacin, Diclofenac sodium | Inhibition of COXs | 65-412.4,
350 |
EPR | Gastrointestinal disorders, kidney failure |
| Biological agents | Etanercept,
Tocilizumab |
Antagonism of TNF-α, Down Regulation of T-cells Activation
|
250,
64.83 |
EPR/Magnetics field | Infection, tuberculosis, gastrointestinal
Infection. |
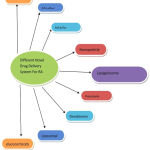 |
Figure 5: Different novel drug delivery system for RA.
|
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
During early stage of rheumatoid arthritis, non steroidal anti-inflammatory drugs such as aspirin, ibuprofen, and naproxen are given to reduce the disease course for long term as it shows promising anti-inflammatory mechanisms without affecting any articular functions. However, it acts by blocking the enzymes such as cycloxygenases COX-1 and COX-2 which plays vital role in reduction of inflammation and pain, & is generated by the prostaglandins. But the drawbacks associated with NSAIDS include renal malfunction, cardiovascular risk and GIT disturbance.7
Glucocorticoids
Currently, near about 45% to 75% of patients are using Glucocorticoids. It acts by releasing phospholipids that leads to reduction of joint inflammation eg. Prednisolone, dexamethasone. Although, these drugs are given during the starting years of treatment however, on frequent & repeated use, it shows several side effects such as hypertension, cardiovascular disease, obesity & sometimes insulin resistance.8
Disease Modifying Anti Rheumatic Drugs (DMARDs)
These are referred as slowing acting anti-RA drugs, first used in the 1980s, which takes nearly 1 to 6 months time period for the treatment and acts by altering RA progressiveness resulting in decreased joint destruction. Due to high efficacy, rapid action, low toxicity, low cost, and ease in administration it has found that Methotrexate is the most commonly used type of DMARDs showing anti-rheumatic activity since last 20 years. Limitations include hepatic cirrhosis, hypersensitivity, allergic reactions and retinopathy.9
Biological Drugs
Biological drugs are regarded as newer DMARDS, which acts by inhibiting overproduction of cytokines (pro inflammatory) in the inflamed site of RA patients. It mainly suppresses the immune system hence, patients are more susceptible to various infections. Currently, these biologic drugs are classified into various categories depending on their targets such as Anti-TNF, IL-1 and IL-6 antagonist-cell co-stimulation blocker & B-cell-depleting agent.10
Various Novel Drug Carrier Systems for Treatment of RA
Since decade ago, different conventional therapies such as analgesics &NSAIDS were used against RA as first line drugs. But with the due course of disease progression they became ineffective & intra-articular steroid deposit is needed to suppress inflammation and pain. With an improved understanding about the role of inflammatory mediators, second line medications which were found to reduce activated T-cell proliferations, various novel drug carrier systems serves as a major breakthrough for treatment RA patients.
Furthermore, it is having wide application in drug delivery as it directly reaches the affected site by controlling the drug release over longer period of time. Several novel therapeutic agents for Rheumatoid Arthritis were summarized in Table 2.
Table 2: Novel therapeutic agents for Rheumatoid Arthritis.
| Types of Carrier | Material | Targeting Group | Therapeutic | References |
| Nanoparticles | PLGA-PEG | — | Betamethasone | 12 |
| PAMAM Dendrimers | Folate | Indomethacin | 13 | |
| Polyester | Ligand for E-selectin | — | 14 | |
| Dendrimers | Paclitaxel | — | Cancer | 15 |
| Fluocinolone | — | Inflammation | 16 | |
| Doxcycline | — | Cancer | 17 | |
| Micelle | PEG Poly(caprolactone) | — | Cyclosporine A | 18 |
| Dextran- Polyoxyethylene Cethyl | — | Cyclosporine A | 19 | |
| Ether(POE-C16 ) | ||||
| Hydroxypropyl- | ||||
| Cellulose(POE-C16) | ||||
| Phospholipids | — | Cyclosporine A | 20 | |
| Cholesterol | ||||
| Liposomes | Phospholipids (with and without covalently linked | — | Clondronate | 21 |
| methotrexate) | ||||
| Cholesterol | — | Methotrexate | 22 | |
| Phospholipids | ||||
| Cholesterol | ||||
| PEG | — | Superoxide Dismulase | 23 |
Nanoparticle
Nanoparticle system mainly depends upon polymers & used as drug delivery system in rheumatoid arthritis therapeutics. Many researchers use PLGA nanoparticles to increase the circulating time and to control the rate encapsulated drug release. It has been observed that PLGA Betamethasone systems are much effective in reducing the inflamed condition than that of free glucocorticoids when administrated intravenous into the arthritic rats and mice. Nagei et al. developed nanogel ointment by bead mill method containing ketoprofen solid nanoparticles having mean particle size of 83nm and reported its anti-inflammatory effect in AIA rats. During RA treatment, FDA used both Gold nanoparticles and metallic nanoparticles. But it prefers metallic nanoparticles mostly for passive targeting in RA. However, gold nanoparticles used for RA treatment shows its non-specific toxicity due to the adverse side effect.11
Several advantages using Nanoparticle drug delivery system includes decreased dosage frequency, increased drug solubility. Prolonged release of drug, Modified pharmacokinetics, tissue distribution of the drug & reduction of toxic side effects. However, it is associated with various limitations as these are costlier, Production is more difficult, Reduce the ability to adjust the dose, Highly sophisticated technology, manufacture skill is required & stability of dosage form is difficult to maintained.
Dendrimers
Dendrimers popularly known as cascade molecules are defined as regularly branched macromolecules with multifunctional surface. It is having characteristic branched structure along with globular shape, which renders huge number of surface groups that can be tailored to provide a template for delivery of drugs thereby increasing the drug loading. Therapeutic effects of dendrimers is been analyzed by using CIA (complete abrogation) mice during synthesis of folic-acid and Methotrexate-conjugate poly (amid amine) dendrimers to the specific targeted activation of macrophages. Methotrexate conjugate with amino groups of dendrimers which are exposed to blood circulation before being delivered into the inflammatory sites showed decreased body weight of CIA mice due to its high cationic charge density.24 Increase drug loading, controlled release, and increased drug delivery to the targeted site are the merits of using Dendrimers whereas High toxicity due to the presence of amino functional group is the drawback of using dendrimers.
Lipogelosome
Lipogelosome is having anti-inflammatory potential as demonstrated by Turker et al. When administrated intrarticularly, the lipogelosome containing Diclofenac sodium, shows anti-inflammatory efficacy as compared to topically administered marketed product of Diclofenac Furthermore, it was reported out from histopathology and biodistribution studies ,that for inflammatory changes in the synovium joints when treated with lipogelosome formulations showed significantly (p<0.05) lower scores than that of contra lateral joints (control).25 It increases retention time & are very effective for the general treatment of joint diseases. But limitations of Lipogelosome include drug loss & issues related to stability.
Emulgels
Emulgels are prepared based on the terms of PH, skin irritation test, stability and in-vitro permeation. Using Carrageenan -induced hind paw edema method in Wistar rats, Vandana et al. developed aloe-vera gel of Nimesulide. Both in-vitro and in-vivo studies were carried out to assess the anti-inflammatory potential of developed formulations. Authors revealed Nimesulide permeation of 54% from Nimesulide emulgels as compared to marketed gel of Nimesulide which is 44% at 30 min, indicating better release of drug without any skin irritation. It also shows higher drug loading capacity of 86% as compared to 70% in case of marketed formulations, hence suitable for having high ant-inflammatory effect.26 Emulgels are effective as they increase drug release at the targeted site with no skin irritation. On contrast, it shows contact dermatitis & poor absorption for larger drug particles as the major drawback symptoms.
Microemulsion
The Micro emulsion is topically administered colloidal dispersions consist of thermodynamically stable system which are administer at the affected inflammation sites. Its particle size ranges from10-100nm and are having very high mean cumulative % values. Researchers argued Tenoxicam micro emulsion formulation showing higher cumulative percent as compared with the conventional cream and suspension of the drug. Tenoxicam (TNX) formulation was prepared by Tween 80, ethanol and oleic acid, used for controlling the inflammation and shows efficient results in oral formulation. Resulted formulations are more effective during topical delivery of Tenoxicam in various inflammatory conditions.27 It is more effective in controlling inflammation and shows higher cumulative percentage values. Furthermore, due to its low viscosity, gelling agent is required.
Micelles
Micelles are the drug delivery system used for treatment of RA .Since, stability of micelles is a major problem to be considered. In order to check the stability and solubility, these were formulated with PEG phospholipids that are been used for drug encapsulation. Activation of synovial macrophages occurs mainly due to over expression of Vasoactive intestinal peptide Receptor. Micelles obtained from phospholipid preparation (phosphogliv) when loaded with methotrexate shows increase capability to decrease inflammation upon administration to arthritic rats.28 Similarly, Camptothecin micelles showed decrease inflammation in comparison to free Camptothecin , when administered to arthritic mice. This method is also useful to improve the solubility of DMARDs .Fewer advantages of using micelles as compared to other NDDS includes good physicochemical stability, increase in the solubility of poorly soluble drug, increase in drug efficiency & Reduced toxicity. But, dilution leading to dissolution is the main drawback.
Liposomes
In order to improve the efficacy of RA conditions, several liposomal systems are been tried which were administered intravenously, getting accumulated in synovial tissue of the patient suffering from RA. Liposomes such as stealth liposomes, cationic liposomes, immuno liposomes, superoxide dismutase liposomes, lactoferrin loaded liposomes & clondronate loaded liposomes were prepared and summarized. The arthritic rats are given phosphatidylcholine and cholesterol based liposome, by encapsulation with clondronate, cause anti-inflammatory therapeutics and are helpful in reducing bone resorption. These liposome clondronate was implemented for depletion of the synovial macrophages. Thus, resultant liposome minimizes joint inflammation and toxicity in contrast to free drugs.29 There are several advantages of liposomes which includes increased efficacy & therapeutic index with decrease in toxicity of the encapsulated agent. Limitations of using liposome were high production cost, fusion of drug molecules, short half -life and decrease solubility.
Liposomal Carrier Systems for RA Treatment
Liposome system is widely used now-a-days for improving the efficacy in rheumatoid arthritis treatments. By intravenous administration, liposomes get accumulated with the synovial tissue of RA patient. It has been observed that cholesterol and phosphatidylcholine liposome encapsulated with clondronate when administered into arthritic rats causes reduction in bone resorption due to anti-inflammatory activity.30 The resultant liposomes shows reduction in joint inflammation & decrease in toxicity. Examples include stealth liposome which causes increased therapeutic efficacy of glucocorticoids that is been frequently administered intravenously by non-specific organ-toxicity. However, stealth liposome and PEG modified liposome which circulates with superoxide dismutase, shows promising drug release.31
However, as summarized in Table 3, different types of liposomal formulations prepared with various phospholipids and by various preparation methods are described which shows improved therapeutic efficacy.
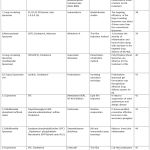 |
Table 3: Different liposomal formulations with various phospholipids along with preparation methods.
|
Conclusion
Despite various advancements in research of RA, neither single drug nor combination therapy has acquired fruitful results. With the advent of various drug delivery strategies as mentioned herein, promises to improve patient outcome by reducing the likelihood of an adverse reaction in conventional therapies. However, to address these emerging issues, researchers have developed nanotherapeutics and liposomal drug delivery system methods providing new idea of RA treatment. These methods due to their controlled release, selective accumulation and reduced systemic toxicity outweighs the conventional RA therapies. The newly developed nanocarriers (liposomal formulations) significantly enhance the therapeutic effectiveness of current drugs for improved remission of arthritis in experimental method.
Future Prospects
With the advancement of knowledge and technology, strategies may be extended in future to facilitate diagnostic imaging and gene therapy which increases the possibility of successfully controlling the progression of disease in all people suffering from rheumatoid arthritis.
Stimuli Responsive Carrier System
The copolymers are applied externally and it consist of large physical and chemical changes that depend on the small stimuli and are been used to control the drug transport and gene delivery that cause some changes in their function and structure of drug release.52
Multifunctional Carrier System
Recently during cancer treatment ,the drug is been delivered which shows the development of various functional carrier system that can also be used for therapeutic release and for diagnostic imaging. Some approaches used for improving the treatment method of rheumatoid arthritis includes.
Folate-conjugated radiopharmaceuticals which are used for targeting into the malignant tissue.
Radiolabel led biologics provide the potential to improve rheumatoid arthritis treatment.
Gene Therapy
This field has grasped the researchers worldwide. Gene therapy consist of nucleic acid that are able to inhibit the cells which causes reduction in the disease promoting protein .It is used mostly for encapsulation with a nuclear factor lipid based nanoparticles, which gets modified with folate and helps in regulating the pro-inflammatory gene expression during the critical component of rheumatoid arthritis.53
Acknowledgments
The authors are very much thankful to the School of pharmaceutical Sciences, Siksha ‘O’ Anusandhan Deemed to be University, for constant help, support and encouragement in academics and research activities.
Conflict of Interest
There is no conflicts of interest.
References
- Dolati S., Sadreddini S., Rostamzadeh D and Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment.Biomedicine & Pharmacotherapy. 2016;80:30-41.
CrossRef - Bonanomi M. H., Velvart M., Stimpel M and Weder H. G. Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis. Rheumatology international. 1987;7(5):203-212.
CrossRef - Dolati S., Sadreddini S and Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomedicine & Pharmacotherapy. 2016;80:30-41.
CrossRef - Liao K. P., Alfredsson L and Karlson E. W. Environmental influences on risk for rheumatoid arthritis. Current opinion in rheumatology. 2009;21(3):279.
CrossRef - Kourilovitch M., Galarza-Maldonado C and Ortiz-Prado E. Diagnosis and classification of rheumatoid arthritis. Journal of autoimmunity. 2014;48:26-30.
CrossRef - Mitragotri S and Yoo J. W. Designing micro-and nano-particles for treating rheumatoid arthritis. Archives of pharmacal research. 2011;34(11):1887-1897.
CrossRef - López A. G. Nanotechnology and autoimmunity. In Autoimmunity. From Bench to Bedside [Internet]. El Rosario University Press. 2013.
- Hoes J. N., Jacobs J. W and Bijlsma J. W. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nature Reviews Rheumatology. 2010;6(12):693.
CrossRef - Van Vollenhoven R. F. Treatment of rheumatoid arthritis: state of the art. Nature Reviews Rheumatology. 2009;5(10):531.
CrossRef - Šenolt L., Vencovský J and Gay S. Prospective new biological therapies for rheumatoid arthritis. Autoimmunity reviews. 2009;9(2):102-107.
CrossRef - Higaki M., Ishihara T., Izumo N and Mizushima Y. Treatment of experimental arthritis with poly (D, L-lactic/glycolic acid) nanoparticles encapsulating betamethasone sodium phosphate. Annals of the rheumatic diseases. 2005;64(8):1132-1136.
CrossRef - Ishihara T., Kubota T., Choi T and Higaki M. Treatment of experimental arthritis with stealth-type polymeric nanoparticles encapsulating betamethasone phosphate. Journal of Pharmacology and Experimental Therapeutic. 2009;329(2):412-417.
CrossRef - Chandrasekar D., Sistla R and Diwan P. V. Folate coupled poly (ethylene glycol) conjugates of anionic poly (amidoamine) dendrimer for inflammatory tissue-specific drug delivery. Journal of Biomedical Materials Research Part A. 2007;82(1):92-103.
CrossRef - Banquy X., Leclair G and Giasson S. Selectins ligand decorated drug carriers for activated endothelial cell targeting. Bioconjugate chemistry. 2008;19(10):2030-2039.
CrossRef - Chen M., Xiao Y., Ping Q and Zong L. Advanced nanomedicine for rheumatoid arthritis treatment: focus on active targeting. 2017.
- Khandare J. J., Jayant S., Singh A., Chandna P and Minko T. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate chemistry. 2006;17(6):1464-1472.
CrossRef - Iezzi R., Guru B. R., Glybina I. V and Kannan R. M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials. 2012;33(3):979-988.
CrossRef - Aliabadi H. M., Brocks D. R and Lavasanifar A. Polymeric micelles for the solubilization and delivery of cyclosporine A: pharmacokinetics and biodistribution. Biomaterials. 2005;26(35):7251-7259.
CrossRef - Francis M. F., Cristea M., Yang Y and Winnik F. M. Engineering polysaccharide-based polymeric micelles to enhance the permeability of cyclosporin A across Caco-2 cells. Pharmaceutical research. 2005;22(2):209-219.
CrossRef - Foong W. C and Green K. L. Treatment of antigen‐induced arthritis in rabbits with liposome‐entrapped methotrexate injected intra. Journal of pharmacy and pharmacology. 1993;45(3):204-209.
CrossRef - Camilleri J. P., Williams A. S and Williams B. D. The effect of free and liposome‐encapsulated clodronate on the hepatic mononuclear phagocyte system in the rat.Clinical & Experimental Immunology. 1995;99(2):269-275.
CrossRef - Williams A. S., Camilleri J. P and Williams B. D. Suppression of adjuvant-induced arthritis by liposomal conjugated methotrexate in the rat. Rheumatology. 1994;33(6):530-533.
CrossRef - Corvo M. L., Boerman O. C., Oyen W. J., Van Bloois L and Storm G. Intravenous administration of superoxide dismutase entrapped in long-circulating liposomes: II. In vivo fate in a rat model of adjuvant arthritis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1999;1419(2):325-334.
CrossRef - Khandare J. J., Jayant S., Singh A and Minko T. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate chemistry. 2006;17(6):1464-1472.
CrossRef - Thakur S., Riyaz B., Kapoor B and Mishra V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomedicine & Pharmacotherapy. 2018;106:1011-1023.
CrossRef - Vandana K. R., Sriramaneni R and Vadlamudi H. C. In-vitro assessment and pharmacodynamics of nimesulide incorporated Aloe vera transemulgel.Current drug discovery technologies. 2014;11(2):162-167.
CrossRef - Goindi S., Narula M and Kalra A. Microemulsion-based topical hydrogels of tenoxicam for treatment of arthritis.AAPS PharmSciTech. 2016;17(3):597-606.
CrossRef - Aliabadi H. M., Brocks D. R and Lavasanifar A. Polymeric micelles for the solubilization and delivery of cyclosporine A: pharmacokinetics and biodistribution. Biomaterials. 2005;26(35):7251-7259.
CrossRef - Bader R. A. The development of targeted drug delivery system for rheumatoid arthritis treatment.Syracuse Biomaterial institute. syracuse university. 2012.
- Williams A. S., Camilleri J. P and Williams B. D. Suppression of adjuvant-induced arthritis by liposomally conjugated methotrexate in the rat.Rheumatology. 1994;33(6):530-533.
CrossRef - Corvo M. L., Boerman O. C and Bloois L. V. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes: II. In vivo fate in a rat model of adjuvant arthritis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1999;1419(2):325-334.
CrossRef - Anderson R., Franch A., Castell M and Pohlers D. Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis research & therapy. 2010;12(4):R147.
- Hofkens W., Grevers L. C., Walgreen B and van Lent P. L. Intravenously delivered glucocorticoid liposomes inhibit osteoclast activity and bone erosion in murine antigen-induced arthritis. Journal of controlled release. 2011;152(3):363-369.
CrossRef - Neog M. K and Rasool M. Targeted delivery of p-coumaric acid encapsulated mannosylated liposomes to the synovial macrophages inhibits osteoclast formation and bone resorption in the rheumatoid arthritis animal model. European Journal of Pharmaceutics and Biopharmaceutics. 2018.
CrossRef - Jia M., Deng C., Luo J., Zhang P and Gong T. A novel dexamethasone-loaded liposome alleviates rheumatoid arthritis in rats.International journal of pharmaceutics. 2018;540(1-2):57-64.
CrossRef - Corvo M. L., Jorge J.C., Crommelin D. J and Storm G. Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2002;1564(1):227-236.
CrossRef - Ulmansky R., Naparstek Y and Barenholz Y. Glucocorticoids in nano-liposomes administered intravenously and subcutaneously to adjuvant arthritis rats are superior to the free drugs in suppressing arthritis and inflammatory cytokines. Journal of controlled release. 2012;160(2):299-305.
CrossRef - Srinath P., Chary M. G., Vyas S. P and Diwan P. V. Long-circulating liposomes of indomethacin in arthritic rats—a biodisposition study. Pharmaceutica Acta Helvetiae. 2000;74(4):399-404.
CrossRef - Sultana F., Neog M. K and Rasool M. Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids and Surfaces B: Biointerfaces. 2017;15:349-365.
CrossRef - Corvo M. L., Boerman W. J. L., Crommelin D. J and Storm G. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes: II. In vivo fate in a rat model of adjuvant arthritis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1999;1419(2):325-334.
CrossRef - Tatheer F., Mazahir R and Kumar S. A. Development and characterization of prednisolone liposomal gel for the treatment of rheumatoid arthritis. Int Res J Pharm. 2015;6:1-5.
CrossRef - Capini C.,Jaturanpinyo M.,Steptoe R., O’Sullivan B.,Davies N and Thomas R. Antigen-specific suppression of inflammatory arthritis using liposomes. The Journal of Immunology. 2009;182(6):3556-3565.
CrossRef - Oelzner P., Bräuer R., Abendroth K and Kinne R. W. Periarticular bone alterations in chronic antigen-induced arthritis: free and liposome-encapsulated clodronate prevent loss of bone mass in the secondary spongiosa. Clinical Immunology. 1999;90(1):79-88.
CrossRef - Elron-Gross I., Glucksam Y and Margalit R. Liposomal dexamethasone–diclofenac combinations for local osteoarthritis treatment. International Journal of Pharmaceutics. 2009;376(1-2):84-91.
CrossRef - Dong J., Jiang D.,Miao L and Huang L. Intra-articular delivery of liposomal celecoxib–hyaluronate combination for the treatment of osteoarthritis in rabbit model. International journal of pharmaceutics. 2013;441(1-2):285-290.
CrossRef - Sultana F., Neog M. K and Rasool M. Targeted delivery of morin, a dietary bioflavanol encapsulated mannosylated liposomes to the macrophages of adjuvant-induced arthritis rats inhibits inflammatory immune response and osteoclastogenesis. European Journal of Pharmaceutics and Biopharmaceutics. 2017;115:229-242.
CrossRef - Chen G., Hao B., and Xia J. Z. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis.Acta Pharmaceutica Sinica B. 2015;5(6):569-576.
CrossRef - Vanniasinghe A. S., Kamali-Sarvestani E., Sharma R., Kumar V., Moghaddam M., Ali M and Bender V. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clinical Immunology. 2014;151(1):43-54.
CrossRef - Van Der Geest T., Laverman P and Boerman O. C. [18] F FDG PET/CT imaging to monitor the therapeutic effect of liposome-encapsulated prednisolone in experimental rheumatoid arthritis. Journal of controlled release. 2015;209:20-26.
CrossRef - Meka R. R., Venkatesha S. H and Moudgil K. D. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. Journal of Controlled Release. 2018;286:279-288.
CrossRef - Ganta S., Shahiwala A and Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of controlled release. 2008;126(3):187-204.
CrossRef - Brown K. D., Claudio E and Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis research & therapy. 2008;10(4):212.
CrossRef - Ganta S., Devalapally H., Shahiwala A and Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery.Journal of controlled release. 2008;126(3):187-204.
CrossRef







