Manuscript accepted on :13-Mar-2019
Published online on: 19-03-2019
Plagiarism Check: Yes
Reviewed by: Sudhan Sundersion George
Second Review by: Dr. Amarendranath Choudhury
M. Ghozali1 , Suhendra Praptama2
, Suhendra Praptama2 , Rini Widyastuti3, Ramdan Panigoro1, Budi Setiabudiawan4
, Rini Widyastuti3, Ramdan Panigoro1, Budi Setiabudiawan4 , Lelani Reniarti4 and M. R. A. A. Syamsunarno*1
, Lelani Reniarti4 and M. R. A. A. Syamsunarno*1
1Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran.
2Faculty of Medicine, Universitas Padjadjaran.
3Faculty of Husbandry, Universitas Padjadjaran.
4Department of Pediatrics, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung.
Corresponding Author E-mail: rizky@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1659
Abstract
Iron overload (IO) because of multiple blood transfusion as a definite therapy for hematological disease with chronic and severe anemia has become a major concern. Deleterious complication contributed by chemically reactive deregulated iron may affect cellular homeostasis systemically lead to tissue and organ damage. When this toxicity occurred in blood cells, alteration of peripheral hematological profile concerning erythrocyte, leucocyte, and platelet most likely to be modified and imperatively need to be evidenced. The experimental IO mice model was established by injecting a low and high dose of iron dextran intraperitoneally. Peripheral erythrocyte, leucocyte and platelet indices measured by hematology analyzer were analyzed. A dynamic tendency of leucocyte absolute cell number and differential cell count of low and high dose iron treatment and a significant decrease of differential monocyte count were found. In addition, high dose iron treatment showed a significantly lower mean platelet volume. In conclusion, this study verified that IO impaired the cellular hematological indices by selectively suppress monocyte number addressing that this mononuclear phagocyte was the most affected immune cell. Furthermore, low mean platelet volume following acquired platelet function defect was evidenced. This research provided an animal experimental model that could be used for further study in finding alternative therapeutic targets on the pathophysiology of iron overload diseases, such as thalassemia.
Keywords
Iron; Monocyte; Platelet
Download this article as:| Copy the following to cite this article: Ghozali M, Praptama S, Widyastuti R, Panigoro R, Setiabudiawan B, Reniarti L, Syamsunarno M. R. A. A. Decrease of Peripheral Monocyte Relative Number and Mean Platelet Volume in Iron Overloaded Mice. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Ghozali M, Praptama S, Widyastuti R, Panigoro R, Setiabudiawan B, Reniarti L, Syamsunarno M. R. A. A. Decrease of Peripheral Monocyte Relative Number and Mean Platelet Volume in Iron Overloaded Mice. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2Ohqg80 |
Introduction
A multiple routine red cell transfusion is a definite lifesaving therapy for thalassemia patients to overcome the severe anemia formed in this inherited hematological disease. Characterized by the reduced or absent production of globin chains of hemoglobin lead to ineffective erythropoiesis and premature hemolysis, this blood system disease is the commonest single gene disorder in the world and causing significant morbidity and mortality in Indonesia and abroad.1 Thalassemia has been a major health concern while becoming one of the catastrophic diseases as in Indonesia with not less than 200,000 children are recorded as thalassemia patients and the number keeps on increasing.2
Iron is an essential yet reactive metal abundantly stored in red cell hemoglobin holds a potent damaging ability to the cell, therefore, it is tightly regulated in our body. The average daily body demand to fit the cell biological metabolism demand is balanced between intake and lost, which is about 1-2 mg.3 Thalassemia major patients who dependently receives the blood transfusion to relieve their severe and chronic anemia, the total body iron is unpreventably accumulated since one unit of transfused blood contains 220-250 mg iron bound to hemoglobin.4 This complication later can trigger the systemic pathological conditions through the production of reactive oxygen species (ROS) that cause tissue damage to lead to organ dysfunction, including the hematological tissue.5,6 Although they are complemented with an iron chelating drug, there is a risk for iron accumulation that can lead to the secondary life-threatening problem in thalassemia major.
Infection constitutes the second most common cause of death after heart failure in thalassemia patients.7 Immune cells and their response are responsible for giving protection from pathogen invasion, adverse disruption of the number and activity of leucocyte were considered to be one of the fundamental notions for thalassemia patients to have an altered immune response, therefore susceptible for infection.7,8 Monocytes, innate immune cells existed as many as 10% of total blood circulating leukocytes, have a pivotal role as regulators and effectors of cellular defense system, among others is the fulfillment of the availability of the macrophages in the tissue that experience infection and inflammation.9 While monocyte-derived macrophage plays a central role in iron metabolism in addition to phagocytosis and immune response, in the case of iron dysregulation, macrophage function can be attenuated.10 Therefore, monocyte in iron overload condition requires further attention.
Traditionally known to have an important role in thrombosis, during a fine balanced restricted inflammation, activated platelet interacts with monocyte and modulates its activity, differentiation into macrophage, and cytokine release.11 However, a defect in platelet aggregation ranging from hypercoagulability and bleeding were identified as thalassemia major patients’ complications.12,13 An in vivo platelet activation prior to its aggregation can be indicated early by a high mean platelet volume, particularly in iron accumulation, this phenomenon needs to be investigated. Combination of continuing premature hemolysis, iron toxicity and tissue injury involving particularly blood system tissue, followed without resolution of inflammation, thalassemia patients are considered to be chronically immune-stimulated therefore more susceptible to infection.14,15 Nevertheless, evidence elaborating the modification of peripheral hematology profile concerning erythrocyte, leucocyte, and platelet affected by iron overload is limited and need for further verification.
The aim of this study is to investigate the effect of iron overload on hematological profile in vivo applying the iron-induced animal model. This study will pave the way to a better understanding of the pathophysiology of iron overload involving erythrocyte, leucocyte and platelet profile.
Materials and Methods
This study was approved by the Ethical Committee on Research of the Universitas Padjadjaran, Bandung, Indonesia No:732/UN6.C.10/PN/2017. All experiments were conducted based on the 3R and 5F principles.
Animals
An experimental study using 21 animal model consists of male white mice (Mus musculus), 8-10 weeks old and body weight approximately 25-30 g, were maintained in cages at room temperature and a 12-hour light/dark cycle with adequate air circulation. Mice were purchased from Bio Farma Company, Bandung, Indonesia. All mice were provided food and water ad libitum.
Grouping of Experimental Group According to the Dosage of Iron Administration
Iron Overload (IO) animal model was established by applying the method as previously described with modification.16 Briefly, mice were divided into 3 groups based on the dose of iron preparation in form of iron dextran (Hemadex-Sanbe, Jakarta, Indonesia) injection. The number of mice was seven for each group:
Group I (control): Saline injection 200 μL/day.
Group II (low dose iron): Hemadex injection 0.05 mg/200 μL/day intraperitoneally.
Group III (high dose iron): Hemadex injection of 0.3 mg/200 μL/day intraperitoneally.
Animals were adapted for one week and the injection was performed daily for 14 days. The dose was calculated to find Animal Equivalent Dose (AED) by multiplying human dose (mg/kg) with Km ratio17. Daily transfusion iron loading in human is 0,34 mg/kg and the Km ratio to convert human dose in mg/kg to AED in mg/kg is multiply human dose by 12.3.17,18
Hematology Assessment
Vacutainer tube containing potassium EDTA (Becton Dickinson, Franklin Lakes, New Jersey, USA) was used to collect peripheral venous blood for hematology profiling. Whole blood was obtained from anesthetized mice via the heart and collected the blood samples in tubes. Automatic hematology analyzer (Sysmex Corp., Japan) was employed to measure 18 whole blood indices.
Statistical Analysis
Multiple group comparisons of treatment differences were performed with the GraphPad Prism program (GraphPad Software, Inc. San Diego, CA). Non-normally distributed data are presented as median with interquartile range (IQR) continued by Kruskal Wallis and followed with Dunn’s multiple comparison test, while normally distributed data as mean with standard deviation (SD) continued by one-way ANOVA and followed with Tukey’s multiple comparison test. A P-value of <0.05 was considered as statistically significant.
Results
To determine whether iron overload affected hematological profile, the value resulted from hematology analyzer (18 indices) was analyzed. An iron overload model was successfully established by injecting with iron dextran and was able to modify hematology change. Iron overload treatments exaggerated the leucocyte, platelet, and erythrocyte indices value. A dynamic tendency of leucocyte absolute cell number and differential cell count of each group of iron treatment was shown in Figure 1. In line with the increased dosage of iron treatment, there was a decreasing tendency of total white blood cell, monocyte, and lymphocyte number within groups, while an increasing trend showed in absolute and differential cell count of granulocyte subset. There was a significantly different effect of iron treatment on differential monocyte count among the groups, using Kruskal-Wallis, X2 = 6.59, P < 0.05. Post hoc pairwise comparison using the Dunn’s test indicated that the median value for the differential monocyte count of the high dose iron treatment group [13.6 (2.6 – 19.1)] was significantly lower than the control [29.7 (17.9 – 31.8)]. However, the median of low dose iron treatment group [14.4 (8.7 – 23.6)] did not significantly differ from the high dose and the control group.
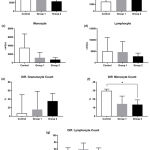 |
Figure 1: Iron Overload affected leucocyte indices.
|
Mice were injected with normal saline in the control group or were injected with iron dextran, respectively, group 1: 0.05 mg/200 μL/day and group 2: 0.3 mg/200 μL/day. (a-d). The absolute count of white blood cell, granulocyte, monocyte, and lymphocyte was presented as the mean with standard deviation. (e-g). The differential count of granulocyte, monocyte, and lymphocyte count was presented as the median with interquartile range. N = 7 mice/group. *P < 0.05 (Dunn’s test).
WBC: white blood cell; Diff.: Differential
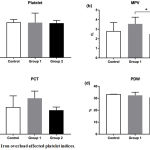 |
Figure 2: Iron overload affected platelet indices.
|
Mice were injected with normal saline in the control group or were injected with iron dextran, respectively, group 1: 0.05 mg/200 μL/day and group 2: 0.3 mg/200 μL/day. (a). The absolute count of platelet was presented as the median with interquartile range. (b-d). The mean platelet value, plateletcrit, and platelet distribution width were presented as the mean with standard deviation.
*P < 0.05 (Tukey’s test)
MPV: Mean Platelet volume; PCT: Plateletcrit; PDW: Platelet Distribution Width
Platelets were affected by iron overload indicated by differential platelet indices i.e. platelet count, mean platelet volume, plateletcrit, and platelet distribution width (figure 2). A significant effect of iron treatment was showed in mean platelet volume among groups, applying ANOVA, [F (2, 17) = 4.263, P = 0.031]. Post hoc comparisons using the Tukey’s test indicated that the mean platelet volume for the high dose iron treatment (2.5 + 0.5) was significantly lower (P = 0.02) than the low dose iron treatment group (3.7 + 0.7). However, the mean platelet volume of the control group (3.1 + 0.9) did not significantly differ from the low and the high iron treatment groups. Figure 3 showed a stationary trend of erythrocyte parameter value resulted from low and high dose iron treatment, even with no significant effect.
Discussion
This study corroborates that the damage caused by iron overload (IO) leads to tissue damage and ultimately to the dysfunction of visceral organs. IO mouse model established in this study makes the effect of IO on the indices of peripheral white blood cells, platelets and erythrocyte profile even more pronounced. The results showed that the IO affected the number and size of blood cells by selectively reducing the differential number of monocytes. In addition, the mean platelet volume is decreased.
The tightly regulated cellular mechanism controls the equilibrium of iron body level between its absorption and demand. Following ingestion, this reactive biometal needs to bind to transferrin and circulate to the intracellular pool, mainly in hepatocytes and macrophages of the reticuloendothelial system as ferritin and in red blood cells (RBC) as a component of hemoglobin.3 Most of the total body iron originated from recycled iron mainly from senescent RBS. The recycling process starts from the engulfment old RBC by macrophages then the iron remains stored or is released back by macrophage into the circulation bound to transferrin for production of new RBC in bone marrow or for storage in hepatocytes4. Unchanged erythrocyte indices after low and high dose iron treatment used in this study imply a careful regulation of iron in maintaining iron homeostasis.
Since humans don’t have the mechanism to eliminate the excess of iron, multiple blood transfusions to overcome the anemia caused by chronic ineffective erythropoiesis happened in thalassemia, inevitably result in IO.4 This endpoint condition gives rise to iron toxicity, a state when the binding capacity of transferrin for iron is surpassed. Ultimately, resulted from non-transferrin bound iron (NTBI) circulating in the blood and subsequently accumulated producing free iron leading to cell and tissue damage until organ dysfunction.3,4
The capacity of white blood cells involved in innate and adaptive body sentinel cells as a response to inflammation and injury is their ability to circulate and migrate from the blood compartment into designated tissue. After stimulated by an inflammation insult, blood circulatory neutrophils accumulate to sites of injury, monocytes migrate into lymphoid and non-lymphoid tissues, while lymphocytes travel from the blood into the lymph through the peripheral lymphoid organs.19 Particularly involving the blood system, IO may harm vascular tissue and reduces hematopoiesis specified by decreasing the number of bone marrow monocyte followed by alteration of platelet indices.5,13,15 Monocytes are provided with instruments i.e. Heme oxygenase, transferrin receptor, and ferroportin, consequently, made it plays a pivotal role in maintaining the body iron balance.20 IO is deleterious since this metal via NTBI is able to catalyze reactive oxygen species (ROS) production which can be an insult in triggering inflammation lead to cell and tissue damage. Previous knowledge has elaborated that the circulatory monocytes chemo-attractively migrate to ROS-induced tissue damage to resolve inflammation while differentiating into macrophage, whereas at the same time granulocyte colony stimulating factor is released by monocyte yielding the increasing number of granulocytes.20 Combine all together, ultimately, thalassemia patients in our previous studies fall into chronically immune-stimulated, since chronic iron loading indicated by the high level of ferritin, increases the oxidative stress lead to persistent inflammation, therefore creating a massive early activated immune cell, particularly involving monocyte and neutrophil.21,22 The limitation of this study that we were unable to determine the vascular damage indicator, the biomarker of immune cells to indicate their activity, serum iron status i.e. ferritin and iron, also oxidative status.
Despite the fact that there is an association of early biomarker indicating the initial activation monocyte with ferritin level in major beta-thalassemia patients,22 a selectively relative decreased of circulating monocyte population evidenced in animal model of this study preliminarily suggests a monocyte compartment shift from circulation to tissue as a competent immune cell in iron balancing to respond the iron overloaded tissue injury. During inflammation due to hypoxia associated with and worsened by iron accumulation oxidative injury, monocytes are activated and differentiated into macrophages.19,20 This unfavorable condition may alter their cytokine release and attenuate macrophage function. However, even with no significant consequence, a decreasing trend was shown in white blood cell and lymphocyte number according to increasing iron treatment concentration. One explanation for these disparate results could be that monocyte and hematopoietic cells undergo extensive cell death in response to ROS in a dose- and time-dependent manner,5,20 therefore the mechanism of these cells disappearance in this study need to be further investigated. Bone marrow haematopoietic cell fate identification applying Graham-Knoll Benzidine staining also needs to be done to see the effect in all blood cells morphologies and stages.
Inflammation related to oxidative burst caused by the excess of iron accumulation, apart from vessel damage and altered coagulation factors involved in homeostasis, can trigger platelets activation. Considering the notion that activated platelet, directly and indirectly, interacts with monocyte and neutrophil lead to these innate immune cells activation,11 persistently activated monocyte, and neutrophil found in our previous studies.21,22 The phenomena most likely may indicate the platelets activation of major thalassemia patients. However, further research investigating the platelet-monocyte aggregation also the PSGL-1, CD11b and CCR2 on the monocyte surface is imperative to provide evidence platelet-monocyte interaction in IO condition.
One of the most commonly used laboratory markers to early characterize platelet function is the mean platelet volume (MPV).13 Increased platelet volume may indicate its greater content in granules, showing a platelet activation also better aggregation and more reactive than the ordinary size one. Limited study and the inconsistent result are available regarding the platelet function study in IO complicated condition. Higher MPV was identified in heterozygous beta-thalassemia patients with no correlation with cardiovascular-related risks.13 However, but in line with this study, a defect in platelet aggregation indicated by a hyporeactivity of platelet after induction with ADP, ristocetin, and collagen was showed in major thalassemia children patients with an iron overload condition.12 The decreased MPV showed in a high dose iron treatment group of this study implies the eventual toxic effect of iron accumulation to platelet function. Still, specific platelet function marker analysis i.e. P-selectin, fibrinogen receptor, and the CD40 ligand is imperative to be explored to understand the fundamental notion of how IO affects the platelet functionality.
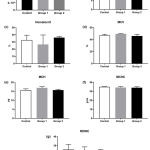 |
Figure 3: Iron overload affected erythrocyte indices.
|
Mice were injected with normal saline in the control group or were injected with iron dextran, respectively, group 1: 0.05 mg/200 μL/day and group 2: 0.3 mg/200 μL/day. (a-f). The absolute count of red blood cell, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration were presented as the mean and standard deviation. (g). The red cell distribution width was presented as the median with interquartile range.
MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RDWC: red cell distribution width.
Conclusion
In summary, this study verified that iron overload impaired the circulating cellular hematological indices by selectively suppress monocyte number addressing that this mononuclear phagocyte was the most affected immune cell. Furthermore, low mean platelet volume following acquired platelet function attenuation was evidenced by iron overload directing that platelet was also affected blood component. This research provided an animal experimental model that could be used for further study in finding alternative therapeutic targets on the pathophysiology of iron overload diseases, such as thalassemia.
Author contributions
Ghozali, Suhendra Praptama, Rini Widyastuti, Lelani Reniarti, Budi Setiabudiawan, Ramdan Panigoro, and MRAA. Syamsunarno conceived the study and participated in the design and data analyses. M. Ghozali, Suhendra Praptama, and MRAA. Syamsunarno were involved in data acquisition and laboratory work. All authors contributed towards drafting and agree to be accountable for all respects of the work. Suhendra Praptama, Rini Widyastuti, Ramdan Panigoro, Lelani Reniarti, Budi Setiabudiawan, and MRAA. Syamsunarno critically reviewed the manuscript. All the authors read and approved the manuscript.
Conflicts of Interest
There is no conflict of interest.
Acknowledgements
This study was funded by Academic Leadership Grant Program Internal Research Grant (ALG), Universitas Padjadjaran 2017. No financial relationships with organizations that have an interest in the submitted research in the previous three years; no other activities or relationships that could affect the submitted research.
References
- Cao A., Galanello R. Beta-thalassemia. Genetics in medicine . official journal of the American College of Medical Genetics. 2010;12(2):61-76.
CrossRef - Ghozali M., Dewi S. P., Ghrahani R., Maskoen A. M., Reniarti L., Sahiratmadja E., et al. Natural resistance-associated macrophage protein 1 gene polymorphisms in thalassemia patients with tuberculosis infection. Paediatrica Indonesiana. 2016;56(2):84-9.
CrossRef - Waldvogel-Abramowski S., Waeber G., Gassner C., Buser A., Frey B. M., Favrat B., et al. Physiology of Iron Metabolism. Transfusion Medicine and Hemotherapy. 2014;41(3):213-21.
CrossRef - Shander A., Cappellini M. D., Goodnough L. T. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009;97(3):185-97.
CrossRef - Chai X., Li D., Cao X., Zhang Y., Mu J., Lu W., et al. ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Sci Rep. 2015;5(1):1-11.
CrossRef - Lu W., Zhao M., Rajbhandary S., Xie F., Chai X., Mu J., et al. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol. 2013;91(3):249-61.
CrossRef - Vento S., Cainelli F., Cesario F. Infections and thalassaemia. The Lancet Infectious Diseases. 2006;6(4):226-33.
CrossRef - Asadov C. D. Immunologic Abnormalities in β-Thalassemia. Journal of Blood Disorders Transfusion. 2014;5(7):1-5.
CrossRef - Davies L. C., Taylor P. R. Tissue-resident macrophages: then and now. Immunology. 2015;144(4):541-8.
CrossRef - Soares M. P., Hamza I. Macrophages and Iron Metabolism. Immunity. 2016;44(3):492-504.
CrossRef - Kral J. B., Schrottmaier W. C., Salzmann M., Assinger A. Platelet Interaction with Innate Immune Cells. Transfus Med Hemother. 2016;43(2):78-88.
CrossRef - Chaudhary H., Ahmad N. Frequency of platelet aggregation defects in children suffering fromg β-thalassemia. Saudi Journal for Health Sciences. 2012;1(2).
- Cikrikcioglu M. A., Celik K., Ekinci I., Nasifov M., Toprak A. E., Cetin G., et al. Mean Platelet Volume in Heterozygous Beta Thalassaemia. Acta Haematol. 2017;137(2):100-5.
CrossRef - Walter P. B., Porter J., Evans P., Kwiatkowski J. L., Neufeld E. J,. Coates T., et al. Increased leucocyte apoptosis in transfused beta-thalassaemia patients. Br J Haematol. 2013;160(3):399-403.
CrossRef - Yu Z. Y., Ma D., He Z. C., Liu P., Huang J., Fang Q., et al. Heme oxygenase-1 protects bone marrow mesenchymal stem cells from iron overload through decreasing reactive oxygen species and promoting IL-10 generation. Exp Cell Res. 2018;362(1):28-42.
CrossRef - Estuningtyas A., Zwicker K., Wahyuni T., Fajri P., Wahidiyat P. A., Freisleben S. K. U., et al. Are Mangiferin and Mangiferin-Containing Plant Extracts Helpful for Iron-Loaded Transfusion-Dependent and Non-Transfusion-Dependent Thalassaemia Patients? Biomedical and Pharmacology Journal. 2018;11(1):29-43.
CrossRef - Nair A. B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27-31.
CrossRef - Taher A., Cappellini M. D., Vichinsky E., Galanello R., Piga A., Lawniczek T., et al. Efficacy and safety of deferasirox doses of >30 mg/kg per d in patients with transfusion-dependent anaemia and iron overload. Br J Haematol. 2009;147(5):752-9.
CrossRef - Porto G. a., Sousa M. D. Iron overload and immunity. World Journal of Gastroenterology. 2007;13(35):4707-15.
CrossRef - Tan H. Y., Wang N., Li S., Hong M., Wang X., Feng Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid Med Cell Longev. 2016;2016(1):1-16.
CrossRef - Ghozali M., Cakranita T. H., Tjahjadi A. I., Reniarti L., Ghrahani R., Syamsunarno M., et al. Fcγ receptor III expression and morphological maturity on neutrophil are associated with higher iron level of major beta-thalassemia. Cellular and Molecular Biology. 2018;64(5):97-101.
CrossRef - Ghozali M., Anggia M. F., Tjahjadi A. I., Reniarti L., Ghrahani R., Syamsunarno M., et al. Positive Correlation between Ferritin and Activated Monocyte in Iron Overloaded Major β-thalassemia Patients. Hiroshima Journal of Medical Sciences. 2018;67(1):78-83.







