Manuscript accepted on :15-Jan-2019
Published online on: 28-02-2019
Plagiarism Check: Yes
Reviewed by: Nicolas Padilla
Second Review by: Akshat Pandey
Final Approval by: Dr. Ayush Dogra
Astrid Feinisa Khairani*1,7 , Muhammad Afif Auliya2, Mas Rizky Anggun Adipurna Syamsunarno3,7, Yunisa Pamela1,7, Kurnia Wahyudi4,7, Nandina Oktavia5,7 and Mohammad Iqbal6,7
, Muhammad Afif Auliya2, Mas Rizky Anggun Adipurna Syamsunarno3,7, Yunisa Pamela1,7, Kurnia Wahyudi4,7, Nandina Oktavia5,7 and Mohammad Iqbal6,7
1Cell Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
2Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
3Biochemistry and Biology Molecular, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
4Department of Public Health, Universitas Padjadjaran, Bandung, Indonesia.
5Anatomy, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
6Department of Cardiology and Vascular Medicine Dr. Hasan Sadikin Hospital, Universitas Padjadjaran, Bandung, Indonesia.
7Research Center of Health System, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
Corresponding Author E-mail: astrid.khairani@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1623
Abstract
The Goldblatt method is one of several methods that can be used in making mouse model of hypertension. The principle of the Goldblatt method itself aims to cause renovascular hypertension by constricting renal blood vessels that results in hypoxic injury and consequently affects the RAAS (Renin-Angiotensin-Aldosterone System). This study aims to determine whether a modification to the Goldblatt method and an additional high salt consumption could be a more effective method in developing a mouse model of hypertension. This study was done on DDY strain mice with 4 weeks of age and 25-30 grams of weight. A simple ligation to constrict renal blood vessel was used as modification from the original Goldblatt 2K1C (two-kidney, one-clip) method. A total of 32 mice were randomized to receive either a modified Goldblatt, modified Goldblatt with salt diet, sham surgery only, or sham with salt diet. Blood pressure was measured at baseline, first week after intervention, and second week after intervention. The data was then analysed by repeated ANOVA method. Hematoxylin and eosin staining was used to analyse histology appearance. This study showed that using a high salt diet only-method generated a hypertensive state faster compared to the modified Goldblatt method, while the modified Goldblatt method produced a steadier increase of blood pressure. Statistically, there was a significant difference of blood pressure between the sham and salt diet group compared to the other three groups at the first week after intervention (p<0,05). The resulting blood pressure from all the methods used in this study was influenced by time. From all four interventions, it is concluded that the modified Goldblatt 2K1C arterial ligation method with an additional high salt consumption had an effect on mice blood pressure. It is prospective to use the salt intervention method for study of hypertension with a short-time period because its acute effect on the rise of diastolic blood pressure was more rapid than the other three groups. On the other hand, the ligation group produced a steadier increase in diastolic blood pressure, therefore might be effective to be used for study of hypertension in a long-time period.
Keywords
Goldblatt 2K1C; Hypertension; Mouse Model; Salt Consumption
Download this article as:| Copy the following to cite this article: Khairani A. F, Auliya M. A, Syamsunarno M. R. A. A, Pamela Y, Wahyudi K, Oktavia N, Iqbal M. A Modified Goldblatt 2K1C Kidney Arterial Ligation Method and Salt Consumption: Which one is Best for Hypertensive Mouse Model?. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Khairani A. F, Auliya M. A, Syamsunarno M. R. A. A, Pamela Y, Wahyudi K, Oktavia N, Iqbal M. A Modified Goldblatt 2K1C Kidney Arterial Ligation Method and Salt Consumption: Which one is Best for Hypertensive Mouse Model?. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2Vsbeyf |
Introduction
Hypertension kills almost 8 million people annually and nearly 1 billion of population have hypertension worldwide.1 Hypertension is a common disease with a high prevalence not only in developing countries but also in developed countries. Research on the pathophysiology of hypertension in human is complex and multifactorial, making it a challenging task.2 Using animal subjects in research of hypertension is an effort to simplify the study of etiology, pathophysiology, complication, and treatment of hypertension.
Several animals can be used as models of hypertension, one of them being mouse. The methods for making such animal models vary, depending on the target of study. Based on past research, Goldblatt method is often used for developing rat models, but rarely used in mouse because of its smaller size.3 The principle of Goldblatt method is producing renovascular hypertension by constricting renal blood vessels that results in hypoxic injury and later affects the RAAS (Renin-Angiotensin-Aldosterone System).2
The original Goldblatt method used silver clips to constrict renal blood vessel.4 In this study, the method was modified by using a simple ligation to constrict renal blood vessel. To achieve hypertension state more rapidly, high salt consumption was also added to the method by using combined salt diet that contains sodium (0,22 mmol/gram) and potassium (0,20 mmol/gram).5 This study aims to determine whether the modified Goldblatt and high salt consumption method is more effective in making mouse model of hypertension. It is prospective that this research could be a future reference for determining an effective method for developing mouse model of hypertension.
Material and Methods
Research Subject and Design
The research design used is experimental laboratory analysis study. The subjects used in this study were DDY strain mouse with 4 weeks of age and 25 – 30 grams of weight, obtained from PT. Bio Farma (Bandung, Indonesia). All mouse adapted to the local air for 3 days after pickup. All mouse were placed in proper cage and given food and drink.
This research was done in Animal Laboratory of Biochemistry and Molecular Biology, Biomedical Sciences Department, Faculty of Medicine Universitas Padjadjaran, Jatinangor and Pharmacology Laboratory of Faculty of Pharmacy Universitas Padjadjaran, Jatinangor.
This study was performed based on 3R principle and was approved by The Research Ethics Committee of Universitas Padjadjaran with the ethical clearance number of 596/UN6.KEP/EC/2018.
Intervention
The mice were divided into four groups based on intervention. The mouse models of hypertension were established using the modified Goldblatt two-kidney, one-clip (2K1C) method through ligation of left renal artery. Following anaesthesia, a ligation on left renal artery was done on group I and II, while sham opening on the left side of each samples was done without left renal artery ligation on group III and IV. High salt solution (Sodium 0,22 mmol/gram and Potassium 0,20 mmol/gram) were given to group II and IV.5,6
Histology Analysis
A total of 12 mice were prepared for kidney dissection. The mice were perfused with formaldehyde 4% to fixate both normal (right) and ligated renal artery (left kidney). Afterward, the kidneys were dissected and soaked in a formaldehyde 4% solution. The kidneys were blocked in paraffin, cut transversally with a thickness of 5 μm, and then stained with Hematoxylin and Eosin (H&E) in the Laboratory of Cell Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung.
The kidney samples were observed under a light microscope using a camera (OptiLab Advance Plus) and images were taken using OptiLab Viewer Plus 3 software. The histological images of the kidneys were processed using ImageJ software version 1.51j8. A total of 1-3 sampling sections were selected from one slide of the mice kidney samples in each group. In each section, images from three cross-sectional areas were taken randomly. The images of the cross-sectional area were taken using 10 × objective magnification.
Blood Pressure Measurement
Blood pressure of the mice was measured without anaesthesia by using “The CODA High Throughput Non-Invasive Blood Pressure”. All samples were measured on the same day every week according to the day the interventions were given.7
Statistical Analysis
The quantitative data obtained was analysed using repeated ANOVA method. The results were considered as significant when p value is < 0.05.
Results and Discussion
Results
From 32 mice enrolled in this study, two of them were dropped out because of death during the data collection period. One excluded sample came from the first group and another was from the second group. Mean blood pressure of the samples is shown on Graph 1.
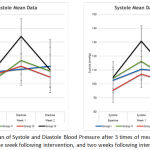 |
Graph 1: Mean of Systole and Diastole Blood Pressure after 3 times of measurement (Baseline, One week following intervention, and two weeks following intervention).
|
Systolic and diastolic blood pressure of each samples was measured at baseline, one week following intervention, and two weeks following intervention. There was no difference among the four groups at baseline and at second week after intervention. There was a significant difference at first week after intervention between sham and salt diet group compared to the other three groups (p<0.0001). Blue line represents group I (mice with modified Goldblatt 2K1C ligation on left renal artery) ; green line represents group II (mice with modified Goldblatt 2K1C ligation on left renal artery and salt consumption); red line represents group III (mice with sham surgery without ligation on left renal artery); black line represents group IV: mice with sham surgery without ligation on left renal artery, with salt consumption.
A total of 32 mice were randomized to receive either ligation on left renal artery, ligation with salt diet, sham only, or sham with salt diet. Blood pressure was measured at baseline, first week after intervention, and second week after intervention. Complete data was available at all time points for 10 mice that received ligation, 10 mice that received ligation with salt diet, 6 mice that received sham, and 6 mice that received sham with salt diet. Mauchly’s test indicated that the assumption of sphericity had been violated, therefore the Huynh-Feldt corrected tests were applied. There was a significant effect of time (p<0,0001). Post hoc comparison on systole and diastole indicated that there was no difference among the four groups at baseline and at second week after intervention. There was a significant difference between sham and salt diet group compared to the other three groups at the first week after intervention. From all four groups, it is concluded that the sham and salt intervention method might be effective for study of hypertension with a short-time period, while the ligation group was steadier and might be used for a long-time period study.
Using mouse as a model of hypertension should mimic the clinical manifestation of hypertension in human because mouse and human share about 98% DNA.8 Based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), hypertension in human is defined as systolic blood pressure over 140 mmHg and/or diastolic blood pressure over 80 mmHg, while a systolic blood pressure between 120 and 139 and a diastolic blood pressure between 80-89 categorized as pre-hypertension.9 Graph 1 shows that at the first week after intervention the fourth group achieved a diastolic hypertensive state, and the second group achieved a diastolic pre-hypertensive state. The first group achieved a prehypertensive state at the second week after intervention.
Discussion
There was a rise in blood pressure at the first week after intervention which then decreased at the second week after intervention in the second, third, and fourth group (ligation and salt diet; sham; sham and salt diet group respectively), but not in the first group (ligation group). It may be concluded that the resulting blood pressure in this study was time-dependent. The ligation group was steadier than the other intervention groups, while the salt group attained a hypertensive state more rapidly.
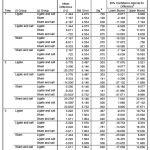 |
Table 1: Result of Systolic Post-Hoc Comparison (p<0,05 = significant).
|
A possible cause of a faster hypertensive state reached by the high salt intervention group is a high concentration of salt in the kidney induced high reabsorption of water to stabilize the osmolarity that resulted in an increased of blood volume. A higher blood volume caused the cardiac output to increase and raised the blood pressure. On the other hand, after some period of time the salt will be removed through urine physiologically. This might explain why on the second week after intervention the blood pressure returned to normal.10
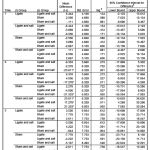 |
Table 2: Result of Diastolic Post-Hoc Comparison (p<0,05 = significant).
|
Renovascular hypertension cause renal ischemia that stimulate recruitment of leukocyte and higher production of reactive oxygen species. Renal ischemia also cause activation of Renin-Angiotensin-Aldosterone-System to happen and stimulates the release of Angiotensin that later on activated by Angiotensin Converting Enzyme into Angiotensin II.2,11 Angiotensin II also considered as inflammatory factor that cause inflammation on renal tissue.12 The inflammatory mechanism was started to appear as infiltration of inflammatory cells on renal tissue as seen on Figure 1. Mice in group II (mice with modified Goldblatt 2K1C ligation on left renal artery and salt consumption) showed some area of infiltration of inflammatory cells. Meanwhile histologic appearance of the other groups were still appeared normal condition. Based on past research, the use of arterial ligation method was considered to be inconsistent and unstable.13 Nevertheless, several other studies have achieved a hypertensive state using this method after 1 week of research.13,14
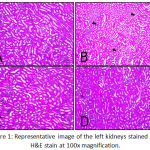 |
Figure 1: Representative image of the left kidneys stained with H&E stain at 100x magnification.
|
Histologic appearance from control (A, upper left), arterial ligation (B, upper right), salt and ligation (C, lower left), and salt only (D, lower right). Pointed arrows indicates infiltration of inflammatory cells.
Being a preliminary study, there were several limitations of this study. Such limitations were the uncontrolled volume of water intake by the mice and physiology of the mice that allowed them to adapt to high salt water consumption.
Conclusion
The resulting blood pressure of all methods used in this study was influenced by time. From all four interventions, it is concluded that the modified Goldblatt 2K1C arterial ligation method with an additional high salt consumption had an effect on mice blood pressure. It is prospective to use the salt intervention group for study of hypertension with a short-time period because its acute effect on the rise of diastolic blood pressure was more rapid than the other three groups. Meanwhile, the ligation group produced a steadier increase in diastolic blood pressure therefore possible to be used for study of hypertension with a long-time period.
Acknowledgements
The authors are grateful to Neni Anggraeni and Irfan Anis Ahmar for the technical assistance.
Conflict of Interest
There is no conflict of interest.
Funding Source
This research was supported by Internal Research Grant Universitas Padjadjaran Riset Fundamental Unpad (RFU) 2018.
References
- Asia S & Region S. E. a. Hypertension fact sheet. World Health Organization. 2011.
- Badyal D. K., Lata H & Dadhich A. P. Animal Models of Hypertension and Effect of Drugs. Indian Journal of Pharmacology. 2003;35:349–362.
- Grossman R. C. Experimental models of renal disease and the cardiovascular system. The open cardiovascular medicine journal. 2010;4:257–264.
CrossRef - Goldblatt H. Studies on Experimental Hypertension: III. the Production of Persistent Hypertension In Monkeys (Macaque) By Renal Ischemia. Journal of Experimental Medicine. 1937;65:671–675.
CrossRef - Costales M., Fitzsimons J. T & Vijande M. Increased sodium appetite and polydipsia induced by partial aortic occlusion in the rat. The Journal of Physiology. 1984;352:467–481.
CrossRef - Warner G. M. et al. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. AJP: Renal Physiology. 2012;302:1455–1464.
CrossRef - Daugherty A., Rateri D., Hong L & Balakrishnan A. Measuring Blood Pressure in Mice using Volume Pressure Recording, a Tail-cuff Method. Journal of Visualized Experiments. 2009. doi:10.3791/1291.
CrossRef - Mural R. J. et al. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science. 2002;296:1661–1671.
CrossRef - Chobanian A. V. et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure The JNC 7 Report. JAMA. 2003;289:2560.
CrossRef - Hall J. E. Guyton and Hall Textbook of Medical Physiology. 2011;(12e). doi:10.1007/s13398-014-0173-7.2.
- Oparil S., Zaman M. A & Calhoun D. A. Pathogenesis of Hypertension. Annals of Internal Medicine. 2003;139:761–776.
CrossRef - Xu Z. et al. Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2). Scientific reports. 2017;7:44911.
CrossRef - Lorenz J. N. et al. Renovascular hypertension using a modified two-kidney, one-clip approach in mice is not dependent on the {alpha}1 or {alpha}2 Na,K-ATPase ouabain-binding site. American journal of physiology. Renal physiology. 2011;346:1–2.
- Chelko S. P., Schmiedt C. W., Lewis T. H., Lewis S. J & Robertson, T. P. A novel vascular clip design for the reliable induction of 2-kidney, 1-clip hypertension in the rat. Journal of applied physiology (Bethesda, Md. 1985;112:362–6.
CrossRef







