Manuscript accepted on :19-Nov-2018
Published online on: 29-11-2018
Plagiarism Check: Yes
Reviewed by: Anusha Dhakshinamoorthi
Second Review by: Ian Martins
Jeanne Adiwinata Pawitan1,2,3 , Tera Dria Kispa1
, Tera Dria Kispa1 , Isabella Kurnia Liem1,3,4
, Isabella Kurnia Liem1,3,4 , Fajar Mujadid1
, Fajar Mujadid1  and Novialdi Novialdi1
and Novialdi Novialdi1
1Stem Cell Medical Technology Integrated Service Unit, Dr. Cipto Mangunkusumo General Hospital/Faculty of Medicine Universitas Indonesia, Jl. Diponegoro 71, Jakarta 10430, Indonesia.
2Department of Histology, Faculty of Medicine Universitas Indonesia, Jl. Salemba 6, Jakarta 10430, Indonesia.
3Stem Cell and Tissue Engineering Research Center, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine Universitas Indonesia, Jl. salemba 6, Jakarta 10430, Indonesia.
4Department of Anatomy, Faculty of Medicine Universitas Indonesia, Jl. Salemba 6, Jakarta 10430, Indonesia.
Corresponding Author E-mail: jeanneadiwip@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1557
Abstract
To compare the viability and characteristics of umbilical cord mesenchymal stromal cells (UC-MSCs) when they were kept in culture in three types of αMEM after confluence for various time points. Viability and characteristic assessments were done after keeping UC-MSC confluent cultures for 0, 24, 48, 72, and 96hours in αMEM with glutamax (Himedia), and without glutamax (Himedia or Gibco).There were thirty cultures, which each ten of them were kept in the three types of basal media, and for each time point and type of basal medium, two flasks were harvested. All viability and characteristic assessments were done after harvest. Viability assessments were done in duplicate, while characteristics assessment by flowcytometry for CD73, CD90 and CD105 were done only once.Further, differences between the various time points in terms of viability and CD73, CD90 and CD105 percentage after various time points were compared and tested by appropriate statistical analysis. Viability was >70% upto 72 hours and 48 hours when the cells were kept in Himediaglutamax containing αMEM and αMEM without glutamax respectively. Flow cytometry showed that CD73, CD90 and CD negative percentage did not differ to initial percentage, but after 24 hours CD105 was slightly decreased to > 60% in Himediaglutamax containing αMEM, while in the two αMEM media without glutamaxthe percentages were below 40%. For our UC-MSC culture, glutamax containing αMEM is better compared to αMEM without glutamax as temporary storage solution, and storage should be restricted to 24 hours.
Keywords
Mesenchymal Stem Cell; Population Doubling Time; Storage Solution; Viability; Umbilical Cord
Download this article as:| Copy the following to cite this article: Pawitan J. A, Kispa T. D, Liem I. K, Mujadid F, Novialdi N. The Potential of Basal Medium Astemporary Prolong Culture of Umbilical Cord Derived Mesenchymalstem Cells. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Pawitan J. A, Kispa T. D, Liem I. K, Mujadid F, Novialdi N. The Potential of Basal Medium Astemporary Prolong Culture of Umbilical Cord Derived Mesenchymalstem Cells. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24446 |
Introduction
We have isolated and propagated umbilical cord derived mesenchymalstromal/stem cells (UC-MSCs) using the multiple harvest explant method,1 and our UC-MSCs met the standards that are set by International Society for Cell Therapy (ISCT) in term of their surface markers and their capacity to differentiate into three lineages, namely adipogenic, chondrogenic, and osteogenic lineages.2Umbilical cord was chosen because itwasa waste of the parturition process and their collection wasnot invasive; therefore, it has many advantages over bone marrow (BM)or adipose tissue (AT), which are previously developed and frequently used as MSC sources. Moreover, UC-MSCshave immune-modulator properties, and are believed to have lower immunogenicity compared to BM or AT MSCs, which privilege them for allogeneic use.3
Our UC-MSCs have been used in clinical trials that are on-going, and after harvest we stored the UC-MSCs in a temporary storage solution at 4-8oC before application. A study showed thatafter harvest, our UC-MSCs could be suspended in high glucose Dulbecco’s modified Eagle’s Medium (DMEM-HG) until 96 hours, which was superior to physiologic saline, and phosphate buffered saline (PBS). However, beyond 96 hours, DMEM-HG stored UC-MSC suspension completely lost their attachment and proliferation capacity, while physiologic saline and PBS stored UC-MSCs still retained some of their attachment and proliferation capacity. Moreover, beyond 96 hours, decline in viability of DMEM-HG was faster compared to physiologic saline stored UC-MSCs.4DMEM-HG contains high glucose, as well as amino acids, vitamins and isotonic ionic concentration, which is similar to human interstitial fluid. 5High glucose containing solution might be supportive to cell metabolism for a certain period, but in longer period might trigger exhaustion in cell metabolism, which led to proliferation arrest.4 Therefore, normal glucose basal medium might be more suitable in maintaining our UC-MSC viability.
Keeping harvested cell suspension in storage solution is often needed due to incompatible harvesting time and stem cell application schedule, either in clinical or preclinical setting, such as in organoid culture, where various types of cells are needed. Another approach to keeping harvested cell suspension in storage solution is keeping near confluent cultures in serum free basal medium until application schedule (prolong culture). Therefore, the aim of this study was to compare the viability and characteristics of our UC-MSCs when they were kept in culture in three types of αMEM after confluence for various time points.
Materials and Methods
This in vitro analyticalstudy was conducted in Stem Cell Medical Technology Integrated Service Unit, RSCM/Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia, and Stem Cell and Tissue Engineering Research Center, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine Universitas Indonesia.Ethical clearancefor this study was obtained from Ethical Commitee of the Faculty of Medicine Universitas Indonesia in 2018(no. 0144/UN2.F1/ETIK/2018).
Sample
The sample waspre-characterizedhuman UC-MSCsat passage-4, whichwas isolated by the multiple harvest explants method, cryopreserved, and stored in liquid nitrogen.1 The sample wasobtained from Stem Cell Medical Technology Integrated Service Unit, RSCM/Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia.
Procedures
Cryopreserved UC-MSCs were thawed quickly and recultured in a 25cm2 tissue culture (T25) flasks in 10% platelet lysate containing complete medium as previously described.6Further, at 80-90% confluence, the cells were harvested and counted.
Comparison of Three Types of Basal Media
Cell suspension was divided in threeequal volumes andthe cell pellets were each resuspended in three types of 10% platelet lysate containing complete media. The three complete media differed in the basal medium,namely glutamax containing αMEM (Himedia AL081G), and αMEM without glutamax (HimediaAL080AorGibco 41061-029). Each cell suspensions in the three kinds of complete media wererecultured into ten T25 flasks to yield thirty T25 flasks.The flasks were observed every day. When the cultures reached 80-90% confluence, complete medium was changed with the respective basal medium, except for two flasks from each basal medium, which was directly harvested and the results were labeled as the results at 0 hour (initial viability). The other flasks, two flasks from each basal medium were kept for 24, 48, 72, and 96 hours in 37oC, and harvested.
After harvesting, viability and characteristic assessments were done. Viability assessments were done in two replications, while characteristic assessments by measuring the CD73, CD90, and CD105 by flowcytometry were done only once.
Data Collection and Analysis
Collected data were viability and percentage of CD73, CD90, and CD105 at harvesting after 0, 24, 48, 72, and 96hours in thethree types ofbasal medium. Viability in the three types of media after various time points were tabulated; mean and standard deviation were calculated and presented as viability curves. Percentage of CD73, CD90, and CD105 in the three types of media after various time points were tabulated, and presented as line graphs.Further, differences between the various time points and the three types of basal media in terms of viability were compared by either ANOVA or Kruskal-Wallis test when the data was unsuitable for ANOVA. Correlation between viability and time were done. All analysis was doneusingstatistical analysis software(SPSS 20.0).
Results and Discussion
By the time, the cell viability was decrease gradually. The gradual decrease of cells’ viability was slowestinthe glutamax containing αMEM (Himedia), followed by αMEM without glutamax(Himedia), and αMEM without glutamax (Gibco) basal media.In the first 24 hours after confluence, the cells’ viability in αMEM without glutamax(Gibco) basal media has decreased until less than 70%; whereas, in the αMEM without glutamax(Himedia) and in the glutamax containing αMEM (Himedia) it decreased in the 72 hours and 96 hours after confluence respectively (Figure 1). Our data were normally distributed, but the variances were nonhomogenous, thus unsuitable for ANOVA. Kruskal Wallis test showed that there were no difference in viabilty between the three tested basal media, and between different time points (p= 0.053).
Characterization of cells using cluster of differentiation (CD) protein markers showed an interesting picture. The expressions were keep stable in CD73, CD90 and CDs negative, however, it was dynamic in the CD105 expression (Figure 2). During the time, expression of CD 90 was constantly >90%, and the CDs negative was constantly below 1%. The expression of CD73 in the three medium was comparable, although in basal media without glutamax (Gibco) it had dropped below 90%. The expression of CD105 could not be maintained in 90% or above. It dropped significantly in the first 24 hours after confluence in the three basal media (Figure 2).
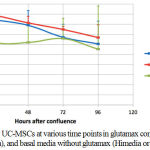 |
Figure 1: Viability of UC-MSCs at various time points in glutamax containing basal media (Himedia), and basal media without glutamax (Himedia or Gibco).
|
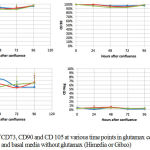 |
Figure 2: Percentage of CD73, CD90 and CD 105 at various time points in glutamax containing basal media (Himedia), and basal media without glutamax (Himedia or Gibco).
|
Our result showed that there were non sigificant decreases in viability between the three tested basal media. However, when we refer to FDA criteria where viable cells for cell therapy should be at least 70% ,7not all of the various time points in those media met the criteria. Before 72 hours after confluence, only in both Himedia with and without glutamax containing αMEM the viabilities were >70%. In αMEM without glutamax (Gibco), viability even decreased below 70% after 24 hours (Figure 1)
Characteriztion after various time points in the three kinds of basal meddia showed that CD73, CD90, and negative mesenchymal stem cell CDs did not differ between the various time points, and media. However, CD 105 dropped below 80% after 24 hours in all media (Figure 2). CD105 (endoglin), which in this study was > 60% after 24 hours of incubation in glutamax containing αMEM, is one of the surface markers on MSCs that should exceed 90% according to ISCT guideline. However, this guideline was set for bone marrow derived MSCs.2Another guideline that was set by International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT)stated that for adipose tissue derived stem cells, CD105 should be >80%..8
CD105 functions as an accessory co-receptor for transforming growth factor beta (TGFβ), which can be found on syncitiotrophoblasts, fibroblast, haematopoietic progenitor cells, and endothelium of microvascular in normal tissues. Its expression is elevated in endothelial cell proliferation, as well as endothelium and stromal cells of solid malignancies, and thus has been suggested to play a role in angiogenesis and neovascularization. Moreover, as TGFβ co-receptor CD105 might play a role in cell adhesion, proliferation, migration, and differentiation.9
In this study, reduced CD105 after 24 hours might interfere with MSC’s differentiation capacity and stemness. In this preliminary study we did not examine MSC’s differentiation capacity or population doubling time and fibroblast colony forming unit as surrogate of stemness, which is a limitation of this study. Therefore, though cell viability still met FDA criteria for cell therapy,7after 72 and 48 hours in both Himedia glutamax containing αMEM and αMEM without glutamax respectively, their functions might be reduced after 24 hours.
Keeping cells in basal media without serum for a certain period is one of the various methods to produce conditioned media rich in growth factors, which was used to treat various pathological conditions. Some studies used hypoxic conditions to produce the conditioned media to increase growth factor secretion. Moreover, normal stem cell niche in tissues is believed to be hypoxic.10 Therefore, for future direction, studies on keeping cell culture in basal medium in hypoxic condition that might prolong the time of storage without interfering cell quality need to be done.
Prolonged culture in basal medium for limited time is very useful when discrepancy of time between cell availability and application schedule is short, either for application in clinical setting or organoid research that needs various types of cells that should be ready in a certain time. Discrepancy between cell availability and application schedule can be managed by cryopreservation, but cryopreservation needs re-culture, which at least needs 7 days to provide cells for application. Moreover, the media after short time storage in hypoxic condition can be utilized and tested as therapeutic agents forvarious pathological conditions.
An alternative instead of to keep cells in culture is to harvest the cells and then keep the cells in a storage solution. We developed a simple processing method to produce adipose tissue derived mesenchymal stem cells (AT-MSCs), 11 and the best storage solution for our AT-MSCs after harvesting was physiologic saline. 12
In conclusion, taken both viability and flowcytometry characteristics into account, for near confluent UC-MSC cultures, glutamax containing αMEM is the best prolong culture solution, and prolong culture should be restricted to 24 hours.
Acknowledgments
Publication of this work was supported by a research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah PUSN 2018, contract no.554/UN2.R3.1/HKP05.00/2018.
Conflict of Interest
There is no conflicts of interest.
Funding Source
A research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah PUSN 2018, contract no.554/UN2.R3.1/HKP05.00/2018.
References
- Pawitan J. A., Liem I. K., Budiyanti E., Fasha I., Feroniasanti L., Jamaan T and Sumapradja K. Umbilical cord derived stem cell culture: multiple-harvest explant method. In.t J. Pharm. Tech Res. 2014;6(4):1202-1208.
- Dominici M., Blanc K. L., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D and Horwitz E. Minimal criteria for defining multi potentmesenchymal stromal cells. The International Society for cellular therapy position statement. Cytotherapy. 2006;8(4):315-317.
CrossRef - Weiss M. L., Anderson C., Medicetty S., Seshareddy K. B., Weiss R. J.,Werff I. V., Troyer D and McIntosh K. R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells. 2008;26(11):2865-2874.
CrossRef - Krishnanda S. I., Agarwal R., Yausep O. E., Rizkita M., Angraeni R and Pawitan J. A. Comparison of Various Solutions for Temporary Storage of Umbilical Cord Derived Mesenchymal Stem Cells. A.R.R.B. 2017;21(2):1-8.
- Life Technologies. DMEM, high glucose. http://www.lifetechnologies.com/id/en/home/technical-resources/media-formulation.8.html. Accessed: July 7. 2015.
- Pawitan J. A., Goei N., Liem I. K and Mediana D. Effect of Cryopreservation and cumulative population doublings on Senescence of Umbilical Cord Mesenchymal Stem Cells. Int. J.Pharm. Tech Res. 2017;10(2):109-113.
CrossRef - US FDA. Guidance for FDA reviewers and sponsors: Content and review of chemistry, manufacturing and control (CMC) information for human somatic cell therapy investigational new drug applications (INDs) (Draft). (http://www.fda.gov/OHRMS/DOCKETS/98fr/03d0349gdl.pdf). Published April 2008.Accessed January 26. 2017.
- Bourin P., Bunnell B. A., Casteilla L., Dominici M., Katz A. J., March K. L., Redl H., Rubin J. P., Yoshimura K and Gimble J. M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648.
CrossRef - Nassiri F., Cusimano M. D., Scheithauer B.W., Rotondo F., Fazio A., Yousef G. M., Syro L.V., Kovacs K and Lloyd R.V. Endoglin (CD105): A Review of its Role in Angiogenesis and Tumor Diagnosis, Progression and Therapy. Anticancer Res. 2011;31:2283-2290.
- Pawitan J. A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014;2014:965849.
- Pawitan J. A., Liem I. K., Suryani D., Bustami A., Purwoko R. Y. Simple lipoaspirate washing using a coffee filter. Asian biomedicine. 2013;7(3):333-338.
- Nofianti C. E., Sari I. N., Novialdi M., Pawitan J. A. Temporary storage solution for adipose derived mesenchymal stem cells. Stem Cell Investig. 2018;5:19.
CrossRef







