Manuscript accepted on :19-Nov-2018
Published online on: 18-12-2018
Plagiarism Check: Yes
Reviewed by: Silvia Lestari
Second Review by: Akmal El-Mazny
Tabark Adel Al-Alousi1, Mousa Mohsin Ali Al-Allak2, Abdulaziz Ahmed Aziz3 and Basima Sh. Al Ghazali4
1Karabala Health Directorate, Ministry of Health, Iraq.
2Obstetrics and Gynecology Department, University of Karbala, Iraq.
3Physiology Department, University of Telafer, Mosul, Iraq.
4Obstetrics and Gynecology Department, University of Kufa, Iraq.
Corresponding Author E-mail: tk_says@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1605
Abstract
Maternal preconceiving nutrition is thought to affect fertility outcomes. The current emphasis on the omega-3 fatty acids, which has been associated with improved fertility in both spontaneous and assisted reproduction conceptions. This study aims to evaluate the role of preconceiving omega 3 polyunsaturated fatty acids supplementation in enhancing the proportion between follicles and retrieved ova, the fertilization rate, and the embryonic grading in subfertile females experiencing intracytoplasmic sperm injection management protocols. One-hundred twenty subfertile women aged 20-40 years-old undergoing intra-cytoplasmic sperm injection were recruited in this randomized double-blinded placebo-controlled clinical trial, at Fertility Center/ Al-Sadr Teaching Hospital/ Al Najaf/ Iraq. They were randomly assigned into two groups; group A (omega-3) includes 60 subfertile women who received one capsule 1000mg omega-3 and Group B (placebo) includes 60 subfertile women who received a placebo contain Liquid Paraffin 500mg for eight weeks. The number of follicles, number of oocytes, fertilization rates, and embryonic quality were recorded in both groups. The study result revealed that the ratio of follicle/retrieved oocyte, the number of metaphase II oocytes, fertilization rate, and grade I embryo were more in the group A compared to group B. Supplementation with Omega-3 polyunsaturated fatty acids can increase the ratio of follicle/retrieved oocyte, the number of metaphase II oocytes, fertilization rate, and grade I embryo, and thereby improving the pregnancy outcome in intracytoplasmic sperm injection cycles.
Keywords
Fertilization Rate; Intracytoplasmic Sperm Injection; Metaphase II Oocytes; Omega-3; Retrieved Ova; Subfertile Women
Download this article as:| Copy the following to cite this article: Al-Alousi T. A, Al-Allak M. M. A, Aziz A. A, Al-Ghazali B. S. The Effect of Omega-3 on the Number of Retrieved Ova, Fertilization Rate, and Embryo Grading in Subfertile Women Undergoing Intracytoplasmic Sperm Injection. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Al-Alousi T. A, Al-Allak M. M. A, Aziz A. A, Al-Ghazali B. S. The Effect of Omega-3 on the Number of Retrieved Ova, Fertilization Rate, and Embryo Grading in Subfertile Women Undergoing Intracytoplasmic Sperm Injection. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=25007 |
ntroduction
Subfertility is a multifactorial disease that affects about 10-15 % of reproductive-aged couple’s world wide.1-3 In Iraq the Primary Infertility was account for about 65.3% and Secondary Infertility 34.7% with ovulation disorders as the main cause of female infertility (41%) while (5%) for tubal obstruction, and only.4 Anovulatory problem is the 15% of the cases had unexplained infertility while abnormality in seminal fluid are the main causes in males.4-5 Some of these fertility problems can be modified while others cannot be changed like age, several studies have reported that chances of getting pregnancy decrease rapidly every year after age 35 years.6-8 Various factors have a remarkable influence on the reproductive capability including changes in lifestyle, level of physical activity, nutrition, and overweight.10-11 Carbohydrates, lipids, and proteins are involved in a wide range of physiological and biochemical activities of the human body. Fatty acids taking a particular interest, which has been linked with improved fertility in both spontaneous and assisted reproduction treatment conceptions.11
The polyunsaturated fatty acids play an important role as a cell membrane component, source of energy, and as signaling molecules.12
It is well-documented that PUFAs supplements can promote general health including adequate fecundability.13 We have Omega-3 and Omega-6 LC-PUFAs, Regarding chemical structure. Omega-3 consists of alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).14-15
Omega-3 fatty acids (omega-3 FA) are precursors of hormones that are produced locally as eicosanoids that are important in the prevention and cure of multiple diseases, especially in women.17-19 In general, eicosanoids derived from-6 PUFA are pro-inflammatory, while those coming from omega-3 are anti-inflammatory.20-21
Omega-3 can influence reproductive processes by acting as precursors for prostaglandin synthesisaswell; omega-3 can modulate the expression of many enzymes involved in prostaglandin and steroid metabolism which are found to be necessary for both ovarian and uterine function and seem to be essential in any successful reproductive procedure.22-25 This study aims to investigate the possible effect of preconceiving supplementation of omega-3 PUFAs on intracytoplasmic sperm injection outcomes on the basis of the ratio between the follicles and retrieved oocytes, the rate of fertilization, and quality of the embryo.
Patients And Methods
Study Design
The current study is a prospective randomized double-blind Placebo-controlled clinical trial, performed at the Fertility Center in Al-Sadr Medical Teaching Hospital, Najaf Al-Ashraf, Iraq, fromJanuary 2017 to February 2018. The study was approved by the Iraqi Medical Ethics Committee in the University of Kufa, Najaf Al-Ashraf/ Iraq.
Study Population
One-hundred twenty subfertile women were randomly selected from subfertile women referred to Al-Sadr Teaching Hospital/ Fertility Center/ IVF Department in Najaf Al-Ashraf City. All women received adequate counseling and signed an informed consent after explanation of the research study aims and steps. Two women were excluded because they did not meet the inclusion criteria and three other did not complete the study. The recruited subjects were randomly assigned into two groups; the Omega 3 group (59 subfertile women) and Placebo group (56 subfertile women) as shown in Figure 1.
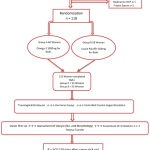 |
Figure 1: Flowchart of the Study.
|
Inclusion Criteria
Women age between 20-40 years-old, Body Mass Index (BMI) between 18-34.99 kg/m2, 1st or 2nd cycle of ICSI, and fresh sperm sample (not aspirated).
Exclusion Criteria
Women who have medical disorders e.g., diabetes mellitus, hypertension or thyroid diseases, sperm collection from epididymal aspiration, Percutaneous Epididymal Sperm Aspiration (PESA) and Testicular Sperm Extraction (TESE), frozen sperm, Consumption of any medications in the last 12 weeks that may influence hormonal assay, history of any diet in the previous three months, tobacco and alcohol consumption, and supplementation of n-3 PUFAs in the past three months.
Baseline Investigations
Blood pressures, random blood sugar, and thyroid-stimulating hormone all were measured before women’s recruitment to the study for the exclusion of hypertension, diabetes mellitus,and thyroid dysfunction respectively.
Omega-3 and Placebo
The women of group A were given omega-3 for eight weeks, while the women of group B were given a placebo for eight weeks before starting the ICSI protocol. The dose of omega-3 was 1000mg one capsule every day composed of 180 mg eicosapentaenoic acid (EPA) and 120 mg docosahexaenoic acid (DHA), the placebo capsule contains 500mg paraffin manufactured by Alzahravi Pharmacology Company. The placebo and omega-3 capsules look similar and cannot be distinguished from each other (same form of package, same shape, same size and color of the capsule). All recruited subjects were followed-up every week by phone messaging, calls and were advised to return the drug pack every one-month visit. At one month, another pack with 30 capsules was supplied to the women. Neither the researcher nor women knew which group takes then-3 or placebo till the end of the research. After eight weeks, all women on day 2 of their cycle will be enrolled in controlled ovarian hyperstimulation depending on standard protocols that are used in fertility center.
Demographic Data Recording, Physical Examination, and Investigation
After recording the routine information, concerning the name, age, address, body weight and height for every woman included in the study, a careful history and physical examination were performed. Transvaginal sonography was performed on day 2 of the cycle to assess endometrial thickness, antral follicle count, and for the exclusion of any uterine or ovarian pathology. Serial transvaginal ultrasound was done for women every 2-3 days till signs of ovulation or ovum pickup for assessment of follicular size and maturation, and for observation of endometrial thickness measurement depending on the day of the cycle.
Hormonal Assays
Hormonal assays were performed, on day 2 ofthe cycle before starting stimulation protocol, 5ml of blood were taken for every woman participated in the study for Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), Estradiol (E2), Prolactin and Thyroid-Stimulating Hormone (TSH) assay. Two days before Human Chorionic Gonadotropin (hCG) administration, other blood samples were collected to measure serum levels of E2 in the same way, 14 days after embryo transfer,a blood sample was collected to measure serum β-hCG level. All Hormonal measurements were done by Mini-vidus technique (using Kit from Bio Merieux SA company/ France).
Ovarian Stimulation Protocols
Two ICSI stimulation protocols were used in this study; the stimulation protocol was selected according to individual patient characteristics.
Short Agonist Protocol (n=42)
On the cycle day 2, Gonadotropins started with Decapeptyle 0.1mg/day. After down-regulation of pituitary GnRH receptors has occurred, the dose of the GnRH agonist is reduced, and the ovulation induction is initiated with recombinant human Follicle Stimulating Hormone (Gonal-f, Merck Serono Specialities Pvt. Ltd., Italy) which contain Follitropinealfa 75 IU/ampoulethese are given subcutaneously, GnRH agonist continued till day of hCG injection.
GnRH Antagonist Protocol (n= 73)
The women received recombinant human Follicle Stimulating Hormone (Gonal-f, Merck) 75 IU/ampoule by daily subcutaneous (SC) injection. GnRH antagonist (Cetrotide 0.25 mg) was SC injected when the diameter of the leading follicle was 14-15 mm (Flexible regime) it administered continuously until the day of hCG administration. The dose of recombinant human Follicle Stimulating Hormone ranged between 150 and 450 IU, based on BMI, the age of the patient, and the anticipated ovarian response and it optimally adjusted depend on the transvaginal ultrasound reports for the number and size of growing follicles.
Ovum Pick Up (DOPU)
Ovulation is induced by hCG administration (intramuscular Pregnyl 10000 I.U. Serono S.P.H, Italy) or by two ampoules (0.1mg) of Decapeptyle (Triptorelin) when the level of E2 >400pg/
ml and two or more follicles reached 17 mm diameter. Then oocytes retrieval was performed under vaginal ultrasound guidance using a transvaginal probe with an ultrasound-guided needle.
The seminal fluid sample was collected at the day of ovum pick up in a private room near the laboratory, after a minimum of 2 days and a maximum of 7 days of sexual abstinence into a clean, wide-mouthed container made of glass or plastic. The eggs are counted by an embryologist in the inverted microscope and stages of oocytes maturity are recorded. Before this done, denudation of the oocytes was performed. Germinal vesicle (GV) and metaphase I (MI) oocytes are excluded and only metaphase II oocytes (MII) are injected with Intracytoplasmic sperm injection.
The fertilization rate was defined as the ratio between the numbers of 2 pronuclei (PN) zygotes to the number of injected MII.FR = (NO.of 2PN on 1st day)/(Total NO.of injected oocytes)× 100.
Each embryo was individually evaluated under the microscope early in the morning of day-2. Embryos were graded based on the following table:
Table 1: Embryo Grading 25
| Grade | Characteristics |
| GradeI | Cells are of equal size; no fragmentation seen |
| GradeII | Cells are of equal size; minor fragmentation only (1–20%) |
| GradeIII | Cells are of unequal size; no fragmentation to moderate fragmentation (21 – 50%) |
| GradeIV | Cells are of equal or unequal size; fragmentation is moderate to heavy (over 50%). |
The most viable zygotes have (PN) of the same size and that is centrally located, with those which have (nuclear percursor body) of same size and number aligned at the pronuclear interphase in preparation for syngamy Under abdominal ultrasound guidance the transfer of embryo was done in the cleavage stage on day 2 or day 3 after oocyte retrieval. the catheter was loaded in the following manner: 15-20µl of transfer medium, 10µl of air, the embryos in 15-20µl of transfer medium, and 10-15 µl of air to seal the catheter (three drops method).
Statistical Analysis
Statistical analysis included mean and standard error,standard deviation, percentage, ratio, chi-square, paired-sample T-test, and student T-test. The data were analyzed using Statistical Package for Social Sciences (SPSS) version 17.0 (SPSS Inc, Chicago, IL, USA). Values were expressed as mean ±SE. A P-value < 0.05 was considered to be statistically significant, P. value <0.01 was considered to be statistically highly significant.
Primary and Secondary Endpoints
Primary endpoint is the embryo quality based on morphological classification as shown in Table (1). Secondary endpoints include oocyte morphology and fertilization rat.
Sample Size
based on a related study (Kermacket al., 2014) the sample size was calculated according to the following formula:
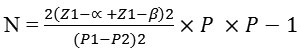
=2*{1.96+0.84} 2*0.15*0.85/{0.28-0.48}
=1.9992/0.04 =49 → 50 Women in each group.
Results
Table (2) displays the general characteristics of subfertile women who have undergone ICSI. The mean age of studied subjects was 29.37 ± 5.51 years (20-39 years), the BMI means of the studied group was 27.84 ± 2.44 kg/m2 (21.7-34.6 kg/m2) and the mean duration of subfertility was 7 ± 3.21 years (2-19 years).
Table 2: Mean and Standard Error of the General Characteristics of the all Participants in the Study (n=115)
| Variable | Mean | Std. error | Range |
| Age/years | 29.37 | 0.49 | 20-39 |
| BMI Kg/m2 | 27.84 | 0.22 | 21.7-34.6 |
| Duration/years | 7.00 | 0.30 | 2-19 |
| No. of retrieved oocyte | 12.22 | 0.56 | 1-33 |
| No. of MII | 8.64 | 0.47 | 1-28 |
| No. of injected oocyte | 7.88 | 0.47 | 1-28 |
| No. of 2PN Zygote | 4.39 | 0.32 | 1-20 |
| No. of follicles | 12.23 | 0.67 | 1-30 |
| Fertilization rate | 60.18 | 2.84 | 7-150 |
| Cleavage stage | 5.77 | 0.34 | 1-18 |
| Cleavage rate | 10.47 | 168.6 | 33-600 |
| Total embryo | 5.53 | 0.32 | 1-17 |
| Grade 1 | 1.42 | 0.17 | 0-11 |
| Grade 2 | 3.36 | 0.24 | 0-11 |
| Grade 3 | 1.83 | 0.14 | 0-12 |
| No. of ET | 2.78 | 0.07 | 0-5 |
| Endometrial thickness mm | 11.12 | 0.12 | 8-18 |
| AFC | 13.20 | 0.27 | 6-19 |
| FSH | 5.13 | 0.18 | 1.2-11 |
| LH | 3.74 | 0.18 | 0.35-10.3 |
| Prolactin | 18.06 | 0.75 | 6.54-56.14 |
| TSH | 2.10 | 0.05 | 0.75-3.41 |
| E2 at day 3 | 35.96 | 1.43 | 0.50-77.51 |
| E2 onthe day of HCG | 2224.23 | 47.04 | 890-3160 |
| Type of infertility | Primary | 107(93%) | |
| Secondary | 8(7%) | ||
| Cause of infertility | Male factor | 102(88.7%) | |
| Female factor | 13(11.3%) | ||
| ICSI trial | 1st | 112(97.4%) | |
| 2nd | 3(2.6%) | ||
| Protocol | Agonist | 42(36.5%) | |
| Antagonist | 73(63.5%) | ||
| Pregnancy test | Positive | 35(30.4%) | |
| Negative | 80(69.6%) |
There were no statistically significant differences in the mean of age, BMI, level of the studied hormones and duration of infertility between the omega-3 group and placebo group as shown in Table (3).
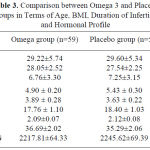 |
Table 3: Comparison between Omega 3 and Placebo Groups in Terms of Age, BMI, Duration of Infertility and Hormonal Profile.
|
Comparison between Omega-3 and Placebo Group Regarding the Number of Follicles, Ratio of Follicles to Oocyte Retrieval, and the Ratio of Follicles No/ No of Injected Oocyte.
The numbers of follicles to the number of injected oocytes in the Omega-3 group were higher than that in the placebo group. However, the difference was not significant (p > 0.05) as shown in Figure (2).
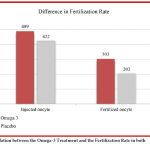 |
Figure 2: Comparisons between Placebo and Omega 3 Group Regarding the Ratio of Number of Follicles / Retrieved Oocytes.Click here to View figure |
The result of the study has shown that the fertilization rate and 2 Pronuclei (2PN) was higher in omega 3 group in comparison with the placebo group (p < 0.05) as shown in (Figure 3).Out of the (489) oocytes injected in the Omega-3 group, (303) were fertilized, whereas only (202) out of (422) injected oocytes in the placebo group were fertilized. Higher rates (p < 0.05) were observed in the Omega- 3 group in comparison to the placebo group.
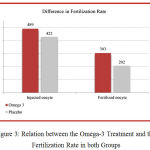 |
Figure 3: Relation between the Omega-3 Treatment and the Fertilization Rate in both Groups.Click here to View figure |
Table (4) Shows a significantly higher number of 2PN zygote (p< 0.01), fertilization rate (p< 0.005), cleavage rate (p< 0.001), grade 1(p< 0.001), and endometrial thickness (p< 0.04) in omega-3 group compared to placebo group. All other parameters show no significant difference between the two groups.
The total number of embryos in the omega-3 group was 6.05±0.47 and in placebo groups 5±0.41 as shown in Table (4). This improvement is the result of the increased number of MII oocytes in the Omega-3 group. However, Omega-3 has not only increases the number of MII oocytes as shown in Figure (4), but it has also improved embryo quality and this is obvious as the number of grade 1 embryo 2.16±0.28 in the Omega-3 group while it is 0.67±0.13 in Placebo group as shown in Table (4).
Table 4: Comparison between Omega3 and Placebo Group Regarding’s ICSI Outcome
| Variable | Omega 3 group (n=59) | Placebo group (n=56) | P-value |
| Mean ±SEM | Mean ±SEM | ||
| No. of follicles | 12.69±0.75 | 11.75±0.79 | 0.390 |
| No. of Retrieved oocyte | 12.91±0.77 | 11.62±0.81 | 0.253 |
| No. of MII | 9.15±0.68 | 8.17±0.64 | 0.306 |
| No. of injected oocyte | 8.28±0.67 | 7.53±0.66 | 0.428 |
| No. of 2PN Zygote | 5.13±0.44 | 3.60±0.46 | 0.019 |
| Fertilization rate | 67.89±3.60 | 51.92±4.20 | 0.005 |
| Cleavage stage | 6.11±0.47 | 5.41±0.49 | 0.304 |
| Cleavage rate | 204.14±9.31 | 134.93±9.31 | 0.001 |
| Total embryo | 6.05±0.47 | 5±0.41 | 0.102 |
| Grade 1 | 2.16±0.28 | 0.67±0.13 | <0.001 |
| Grade 2 | 3.33±0.34 | 3.48±0.34 | 0.769 |
| Grade 3 | 0.61±0.15 | 1.08±0.25 | 0.100 |
| No. of ET | 2.93±0.08 | 2.73±0.11 | 0.159 |
| Endometrial thickness mm | 11.37±0.12 | 10.87±0.21 | 0.045 |
| AFC | 13.16±0.36 | 13.28±0.42 | 0.838 |
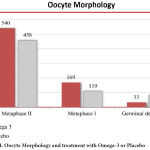 |
Figure 4: Oocyte Morphology and treatment with Omega-3 or Placebo.
|
The total pregnancy rate (positive pregnancy test) in both groups was 35 women (30.4%) while 80 women had a negative pregnancy test. Out of 59 Women in Omega-3 group, 20 (33.9%) women developed pregnancy where in placebo group only 15(26.8%) women had positive pregnancy tests. However, the differences were statistically not significant as shown in Table (5).
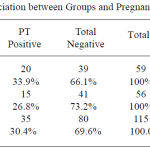 |
Table 5: Association between Groups and Pregnancy Test Result.
|
Table (6) was done to exclude the effect of ovarian stimulation protocols, as 73 subfertile women undergoing antagonist protocol while only 42 subfertile women in placebo but there was no significant difference on their effects on both studies groups.
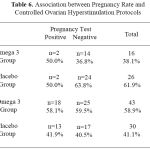 |
Table 6: Association between Pregnancy Rate and Controlled Ovarian Hyperstimulation Protocols.
|
Discussion
The current study was conducted to clarify the impact of omega 3 PUFAs supplementation on ICSI clinical outcomes, by comparing the main ICSI parameter like the number of retrieved ova, the fertilization rate, and the embryonic grading in two groups of subfertile women experiencing ICSI management protocols. One group receives omega 3 PUFAs supplementation and the other group receiving placebo for 56 days (8 weeks). The study attempts to ameliorate the effects of certain important confounders that may affect the result like BMI and age of participant by selecting the subfertile women with younger age and canceling obese women from the study and by matching such variables between the two groups.26
Fifty-seven (96.6%) out of 59 subfertile women in the Omega-3 group have their 1stICSI trial, while 55 (98.2%) out of 56 subfertile women in the placebo group were with 1st ICSI trial. Only 3 women have the second trial of ICSI. There were no significant differences between the two groups regarding the number of ICSI trial. This finding probably excludes the effect of a number of trials on ICSI results. Sixteen (27.1%) out of 59 subfertile women in the Omega-3 group follow agonist protocol and 43(72.9%) follow antagonist protocol, wherein Placebo group 26(46.4%) women follow agonist protocol and 30(53.6%) women follow antagonist protocol. The results of our research study were in harmony with other research studies that concluded the antagonist protocol was shown to be an easy and safe protocol.27-29
Regarding the type and causes of infertility high percentage of subfertile cases presented with primary subfertility 107(93%) and only 8 (7%) with secondary subfertility, male factor carried a higher percent 102 (88.7%) than other causes that indicated for ICSI. This finding supported by other research studies who found that malefactor of subfertility is predominant than other causes (56.43%).30-31 The possible explanation for the high incidence of infertility among Iraqi men is that our country environment suffered from many profanations as result of many wars.32
Despite the pregnancy rate reflects the success of the ICSI process, it is better to use live birth rate, better to use live birth rate but this needs more time and follow up. For this reason, we considered the embryo quality to be the primary endpoint.
The numbers of follicles in the omega-3 group were significantly higher than that in the Placebo group, and there is a higher number of the retrieved oocyte in the Omega-3 group these findings agree with other findings in prior studies who reported a high intake of dietary fish oil led to a significant increase in the ovulation and follicles development.33
The result of this study also revealed a significant increase in the numbers of the embryo in the Omega-3 group in comparison with the Placebo group. A possible explanation may be due to the fact that PUFAs supplementation associated with suppression of production of PGF2 by compacting with prostaglandin endoperoxide synthase (PGHS) enzyme that required for the change of the Arachidonic acid to the PGF2. It is believed that decreased PGF2 level, elevating the chance of preserving the life of the newly developed embryo.34-36 This agrees with Hammicheand Kermack they reported that assessing preconception supplementary n-3 PUFA that results in improving embryo morphology and ART outcomes.37-38
Conclusion
These findings suggest that supplementation with Omega-3 FAs in IVF techniques could positively influence the final result of the reproductive sequel. However further research studies are required considering racial and ethnic differences response to omega-3 FAs in order to permit future Meta-analysis performance and application in clinical guidelines of management.
Acknowledgements
The authors are grateful to all staff in the Department of Physiology, College of Medicine, University of Kufa, Iraq,they are grateful also to all staff in the Fertility Center, Al-Sadr Teaching Hospital/ Al Najaf, Iraq.
Conflicts of Interest
There is no conflicts of interest.
References
- Agarwal A., Mulgund A., Hamada A and Chyatt R. M. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37.
CrossRef - Boivin J.,Bunting L., Collins J. A and Nygren K. G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2002;22(6):1506-12.
CrossRef - Yi-Ling H., Bih-Jaw K. Evaluations of Emotional Reactions and Coping Behaviors as Well as Correlated Factors for Infertile Couples Receiving Assisted Reproductive Technologies. Journal of Nursing Research. 2002. doi: 10.1097/01.JNR.0000347610.14166.52.
CrossRef - Ali M. M.,Ghazi W and Abboud A. H. Prevalence of Anovulation in Subfertile Women in Kerbala 2012, a descriptive Cross-Sectional Study. Print ISSN: 2073-8854 & Online ISSN: 2311-6544. Magazin of Al-Kufa University for Biology. 2014;6:2.
- Razzak A. H., Wais S. A. The Infertile couple: A cohort study. Eastern Mediterranean Health Journal. 2002;8(2/3).
- Rowe T. Fertility and a woman’s age. J Reprod Med. 2006;51(3):157–63.
- Homan G. F.,Davies M and Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatments: a review. Hum Reprod Update. 2007;13(3):209-23.
CrossRef - American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male a committee opinion. Fertil Steril. 2012;98:294-301.
CrossRef - Huang H., Hansen K. R., Factor-Litvak P., Carson S. A., Guzick D. S., Santoro N., et al. Predictors of pregnancy and live birth after insemination in couples with unexplained or malefactor infertility. Fertil Steril. 2012;97(4):959-967.
CrossRef - Esmaeilzadeh A. D., Basirat S. H. Physical activity and body mass index among women who have experienced infertility. Arch Med Sci. 2013;3:499-505.
CrossRef - Azadeh N.,Dehghani-Firouzabadi R., Daneshbodi H.,Lotfi H. M.,Vaziri N., Mozaffari-Khosravi H. Effect of Omega-3 Supplementation on Visfatin, Adiponectin, and Anthropometric Indices in Women with Polycystic Ovarian Syndrome. J Reprod Infertil. 2015;16(4):212-220.
- Ashlee H., Anne R D., Joo Y. O. H., Aimee L., Victor M. Cell signaling by reactive lipid species: New concepts and molecular mechanisms. Biochem. J. 2012;442:453-464.
CrossRef - Lisa J. M.,Tsagareli V., Noakes M and Norman R. Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation. Nutrients. 2016;8:10. doi: 10.3390/ nu8010010.
- Muskiet F. A., van Goor S. A., Kuipers R. S., Velzing- Aarts F. V., Smit E. N., Bouwstra H., et al. Long-chain polyunsaturated fatty acids in maternal and infant nutrition. Prostaglandins Leukot Essent Fatty Acids. 2006;75:135–44.
CrossRef - Chilton., Dutta R., Reynolds M. L., Sergeant S., Mathias A. R and Seeds C. M. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients. 2017;9:1165. doi: 10.3390/nu911116.
- Patterson E.,Wall R., Fitzgerald G. F., Ross R. P and Stanton C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. Journal of Nutrition and Metabolism. 2012;16. Article ID 539426, doi:10.1155/2012/539426.
CrossRef - Dess M.,Noce A., Bertucci P.,di Villahermosa M. S., Zenobi R.,Castagnola V., Addessi E and Di Daniele N. Atherosclerosis, Dyslipidemia and Inflammation: The Significant Role of Polyunsaturated Fatty Acids. ISRN Inflammation. 2013;13. Article ID 191823,http://dx.doi.org/10.1155/2013/191823.
CrossRef - Saldeen P and Saldeen T. Obstet Gynecol Surv. Women and omega-3 Fatty acids. 2004;59(10):722-30. quiz 745-6.
- Ricciotti E and G. A. Fitzgerald. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis and Vascular Biology. 2011;31(5):986–1000.
CrossRef - Tai C. C and Ding S. T. “N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: mechanisms and implications for obesity prevention.”Journal of Nutritional Biochemistry. 2010;21(5):357–363.
CrossRef - Robinson R. S., Pushpakumara P. G., Cheng Z., Peters A. R., Abayasekara D. R. Wathes Effects of dietary polyunsaturated fatty acids on ovarian and uterine function in lactating dairy cows. Reproduction. 2002;124(1):119-931.
CrossRef - Claire W. D., Robert E. A. D., John A. R. Polyunsaturated fatty acids in male and female reproduction. Biology of Reproduction. 2007;77(2):190-201.
CrossRef - Wonnacott K. E., Kwong W. Y., Hughes J., Salter A. M., Lea R. G., Garnsworthy P. C., Sinclair K. D. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction. 2010;139(1):57-69.
CrossRef - Lazzarin N., Vaquero E., Exacoustos C., Bertonotti E., Romanini M. E., Arduini D. Low-dose aspirin and omega-3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertil Steril. 2009;92(1):296-300.
CrossRef - Hazlet D. Embryo fragmentation what does it mean Embryology, Infertility and IVF: embryo, embryology lab, evaluating egg quality. 2011.
- Liu H., Zhao H., Yu G.,Li M., Ma S., Zhang H.,Wu K. Conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI): which is preferred for advanced age patients with five or fewer oocytes retrieved? Archives of Gynecology and Obstetrics. 2018;297(5):1301–1306.
CrossRef - Coccia M. E., Comparetto C., Bracco G. L., Scarselli G.,antagonists G. R. H. European journal of obstetrics, gynecology and reproductive biology. 2004;115(10)S44-56.
- Bodri D., Sunkara S. K., Coomarasamy A. Gonadotropin-releasing hormone agonists versusantagonists for controlled ovarian hyperstimulation in oocytedonors: A systematic review and meta-analysis. Fertility and sterility. 2011;95(1):164-169.
CrossRef - Al-Inany H. G., Youssef M. A and Aboulghar M et al. GnRH antagonists are safer than agonists: an update of a Cochrane review. Hum. Reprod. 2011;17(4):435.
- Al-Turki H. A. Prevalence of primary and secondary infertility from tertiary center in eastern Saudi Arabia. Middle East Fertility Society Journal. 2015;20(4):237-240.
CrossRef - Al-Ghazali B. S and Al-Jarrah D. M. Factors Affecting Intra-Cytoplasm Sperm Injection (ICSI) and Pregnancy Outcome in the Fertility Center of Al –Najaf City. The Iraqi Postgraduate Medical Journal. 2013;12. SUPPLEMENT.
- HFEA. Human Fertilisation and Embryology Authority, code of practice, 8th Edition. 2009.
- Fleming R., Seifer D. B., Frattarelli J. L., Ruman J. Assessing ovarian response: Antral follicle count versus anti-Mullerian hormone. Reprod Biomed Online. 2015;31:486–496.
CrossRef - Ferraretti A. P., Marca A. L., Fauser B. C., Tarlatzis B., Nargund G., Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum Reprod. 2011;26:1616–1624.
CrossRef - Ray A., Amit S., Anil G., Roy H. Unexplained infertility: An update and review of practice. Reproductive BioMedicine Online. 2012;24:591– 602.
CrossRef - Kazemi A., Fatemeh R., Mohammad H. N., Ali A. S. Y., Mehdi A. Does dietary fat intake influence oocyte competence and embryo quality by inducing oxidative stress in follicular fluid. Iran J Reprod Med. 2013;11(12):1005-1012.
- Hammiche F., Marijana V., Willeke W., de Vries H. M. J ., Nick S., Macklon J. S. E. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertility and Sterility. 2011;95(5):1820-1823.
CrossRef - Alexandra J. K.,Calder C. P.,Houghton D. F., Godfrey M. K and Macklon S. N. A randomised controlled trial of a preconceptional dietary intervention in women undergoing IVF treatment (PREPARE trial). Kermack et al. BMC Women’s Health. 2014;14:130 Page 2 of 7 http://







