Manuscript accepted on :26-June-2018
Published online on: 26-07-2018
Plagiarism Check: Yes
Reviewed by: Medvedev Ilya Nikolaevich
Second Review by: Kulvinder Kaur
Final Approval by: Dr Ayush Dogra
Gamal S. Abd El-Aziz1 , Hesham N. Mustafa1
, Hesham N. Mustafa1 , Hamid Abdulraouf Saleh1
, Hamid Abdulraouf Saleh1 and Magdy M. O. El-Fark2
and Magdy M. O. El-Fark2
1Department of Anatomy, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
2Department of Anatomy, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
Corresponding Author E-mail: hesham977@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1500
Abstract
This study was designed to address the protective effects of Zingiber officinale on the toxic outcomes of prenatal Cadmium administration on pregnancy outcome. Pregnant female Sprague-Dawley rats were randomly divided into four groups (eight rats/each), control group received distilled water, 2nd group treated with 8.8 mg of CdCl2/kg b. wt, 3rd group treated with 250 mg of Zingiber officinale/kg b. wt, and 4th group treated with 250 mg of Zingiber officinale/kg b. wt, followed by 8.8 mg of CdCl2/kg b.wt. Daily body weight of pregnant was recorded from GD1-GD20, and then pregnant rats were sacrificed at GD20. Samples of maternal and fetal livers and kidneys were processed for histological examination. Administration of Cd to pregnant rats showed adverse effects on pregnant mothers and their fetuses; reduced maternal weight gain, reduced absolute organ weights, reduced fetal growth parameters and placental weights together with altered histological appearance of the maternal and fetal livers and kidneys. While co-administration of Zingiber officinale showed an improvement of these toxic alterations. Zingiber officinale through its antioxidant activity could be beneficial against toxic outcomes of Cd exposure during pregnancy.
Keywords
Cadmium; Fetal Toxicity; Maternal Toxicity; Zingiber Officinale
Download this article as:| Copy the following to cite this article: El-Aziz A. G. S, Mustafa H. N, Saleh H. A, El-Fark M. M. O. Zingiber Officinale Alleviates Maternal and Fetal Hepatorenal Toxicity Induced by Prenatal Cadmium. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: El-Aziz A. G. S, Mustafa H. N, Saleh H. A, El-Fark M. M. O. Zingiber Officinale Alleviates Maternal and Fetal Hepatorenal Toxicity Induced by Prenatal Cadmium. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=21727 |
Introduction
The undesirable impacts of heavy metals on the women health during pregnancy have acquired increased attention during recent years.1 Cadmium (Cd) is considered industrial pollutant and toxic environmental.2-5
Cd has 20 – 30 years half life that is attributed to its low excretion rate from the body.6-8 Studies showed that Cd induced hepatotoxicity, lung damage, testicular damage and nephrotoxicity.9-12
Its reported that women are vulnerable to Cd toxicity, that is attributed to increased intestinal uptake, which is prevalent in women than in men.13,14 During pregnancy, Cd exposure could promote the development of pregnancy complications e.g. spontaneous abortion, toxaemia and anaemia.15 Experimental studies in pregnant animals have found a variety of adverse reproductive outcomes like decreased litter size, increased resorptions and foetal death, growth retardation and different congenital malformations in offspring of Cd exposed animals.16
Many mechanisms have explained the Cd-mediated toxicity; one of these is related to the alterations in oxidative status.17 Studies confirmed that Cd-induced oxidative stress is attributed to increased lipid peroxidation,10 which had been shown to stimulate intracellular ROS (reactive oxygen species) production due to mitochondrial membrane disruption, that is the main target of the cellular effect.18 Cellular damage appears when ROS generation exceeded that of decomposition due to antioxidant defense.19 Also, it is known that pregnancy is a condition that favors oxidative stress due to rich mitochondrial component in the placenta,20 that contribute to excessive ROS production which affects development and growth of the fetuses.21,22
A growing concentration focusing on the biological activities of medicinal herbs, cause of few side effects, natural origin and cost effectiveness. Zingiber officinale Roscoe from the Zingiberaceae family, is broadly used as a spice and a traditional medicine. Zingiber officinale bioactive molecules have a potent antioxidant activity.23-25 In-vivo and in-vitro tests are done to study the anti-oxidative properties of Zingiber officinale and its components; these conclude that strengthening the defense mechanisms of the body will protect the body against different diseases by improving the antioxidant status.26 Experimental work showed that Zingiber officinale significantly elevated antioxidant enzymes levels and lowered the induced lipid peroxidation that is accompanied by reduced glutathione (GSH), and GSH-dependent enzymes glutathione peroxidise.27
This study therefore, was designed to address the protective effects of Zingiber officinale co-administration on the different parameters of pregnancy outcome plus the histopathological changes of the maternal and fetal livers and kidneys after in utero Cd administration.
Materials and Methods
Chemicals
Cadmium chloride (CdCl2) and all other chemicals used in this study were purchased through local agents, Jeddah, KSA, while ginger rhizomes were purchased from local markets of Jeddah. CdCl2 is dissolved in distilled water to prepare the solution to a concentration of 8.8 mg/ml (10% of LD50).28 Zingiber officinale was prepared according to Kamtchouing et al,29 where ginger rhizomes were dried at room temperature, and crushed to 50 g powder then dissolved in 1000 ml of distilled water, then filtered to obtain the aqueous extract. The extract concentration is 50 mg/ml equal to 250 mg/kg.
Animals and Mating
Nulliparous adult female Sprague-Dawley albino rats (weighing 175-200 g) obtained from the Animal House. During the study, the female rats were kept in metallic cages under standard temperature (24 ± 2ºC), humidity (55 ± 5%) and lighting (12h light: 12h dark) conditions. Rats fed a standard diet ad libitum and had access to water. Mating was assisted by placing the individual females overnight in the home cage of a singly-housed male of the same stock. Mating was confirmed by vaginal lavage smear that detect positive identification of spermatozoa and is considered as gestation day 1.
Committee of Animal Investigations in Anatomy department, Faculty of Medicine has approved by the study. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal Treatment
The female pregnant rats were divided randomly into four groups of eight rats each and all treatments given through oral intragastric tube in the drinking water during only gestation (Prenatal study) and during gestation and lactation periods (Postnatal study). Cd and Zingiber doses and manner of administration were chosen on the basis of previous studies30-32 as follows:
Group I (control group): received distilled water only.
Group II (Cd group): received 8.8 mg of CdCl2/kg b.wt.28
Group III (Zingiber officinale group): received 250 mg of Zingiber officinale/kg b.wt.29
Group IV (Cd+Zingiber officinale group): received 250 mg of Zingiber officinale/kg b. wt., followed by 8.8 mg of CdCl2/kg b.wt.
Evaluations of Pregnant Females
The pregnant rats of each group were observed daily throughout the gestation period for body weight. The pregnant rats of different groups were anesthetised and sacrifced by decapitation on 20th day of gestation. After laparotomy, the liver and kidneys were obtained, and their weights were recorded.
Morphological Studies
Small pieces from liver and kidney of the mothers and fetuses were taken immediately and immersed in 10 % buffered neutral formalin. Serial sections (5 µm thickness) were cut and stained by Haematoxylin and Eosin and examined using an Olympus BX53 microscope equipped with a DP73 digital camera (Olympus, Tokyo, Japan).
Statistical Analysis
Quantitative data were represented as mean ± standard deviation of different parameters for the treated groups. One-way ANOVA (analysis of variance) with Bonferroni Post Hoc for the means of all quantitative data were done. Fisher Exact Probability test was used for rat embryolethality. The significance level for all comparisons was set at p< 0.05. Statistical analyses were performed by using GraphPad Prism v.5 software (GraphPad, San Diego, CA).
Results
Effects on Maternal Weight Parameters
As seen in Table (1), the mean values of initial body weight of all animals were equal. Regarding other parameters including, body weight, gravid uterine weight, and placental weights, the results showed that these parameters were approximated in both control and Zingiber officinale treated groups without any significant difference. In Cd treated group, there was a significant decrease in these parameters when compared to control group, Zingiber officinale treated group, and Cd+ Zingiber officinale treated group. However, co-administration of Zingiber officinale together with Cd resulted in a noticeable improvement in all the values towards the control figures.
Table 1: Effect of Cd and Zingiber officinale on maternal weight parameters and placental weight.
| Groups | Initial body Weight (g) (n=8) | Final Body Weight (g) (n=8) | Gravid uterine Weight (g) (n=8) | Placental Weight (g) |
| Control group | 195.3 ± 8.9 | 302.1 ± 11.8 | 57.9 ± 3.6 | 0.68 ± 0.06
(n=85) |
| Cd group | 192.6 ± 8.3 | 255.5 ± 10.7 a,b,c | 36.2 ± 3.6 a,b,c | 0.47 ± 0.1 a,b,c
(n=69) |
| Zingiber officinale group | 196.3 ± 9.3 | 301.6 ± 13.1 | 57.4 ± 3.5 | 0.67 ± 0.07
(n=82) |
| Cd+Zingiber officinale group | 198.0 ± 8.8 | 287.4 ± 10.9 | 47.2 ± 3.3 a,b | 0.59 ± 0.11 a,b
(n=76) |
ANOVA (Bonferroni Post Hoc) test: results are expressed as Mean±SD
a- P <0.0001 compared to Control group.
b- P <0.0001 compared to Zingiber officinale treated group.
c- P <0.0001 compared to Cd-Zingiber officinale treated group.
Effects on the Weights of Maternal Internal Organs
Regarding relative weights of both liver and kidney, it was noticed that the values from both Cd and Cd+Zingiber officinale treated groups were approximated to both control and Zingiber officinale treated groups (Table 2).
Table 2: Effect of Cd and Zingiber officinale on maternal liver and kidney.
| Groups | Relative maternal liver Weight (g) (n=8) | Relative maternal kidney Weight (g) (n=16) |
| Control group | 4.5 ± 0.24 | 0.45 ± 0.03 |
| Cd group | 4.2 ± 0.16 | 0.39 ± 0.04 |
| Zingiber officinale group | 4.4 ± 0.2 | 0.43 ± 0.04 |
| Cd+ Zingiber officinale group | 4.3 ± 0.17 | 0.41 ± 0.05 |
ANOVA (Bonferroni Post Hoc) test: results are expressed as Mean±SD
Histological Changes of the Maternal Liver of Different Groups
Microscopic examination of liver sections obtained from both control and Zingiber officinale treated groups, revealed normal histological architecture of the liver.
In Cd-treated group, microscopic examination showed markedly congested and dilated central vein with detached endothelial lining. In addition, dilatation of blood sinusoids and inflammatory cellular infiltration were observed different locations of the hepatic lobule. Most of the hepatocytes in the hepatic lobule exhibited variable degrees of fatty and hydropic degeneration. Furthermore, nuclear changes in some hepatocytes in the form of karyolysis or pyknosis were detected. Dilation and congestion of the portal venules with inflammatory cellular infiltration were detected.
In Cd+ Zingiber officinale treated group, microscopic examination revealed that nearly similar picture to the control liver. However slight congestion of portal venule, dilatation of the blood sinusoids, less marked hydropic degeneration, some hepatocytes showed karyolsed nuclei and some inflammatory infiltrates were observed (Figure 1).
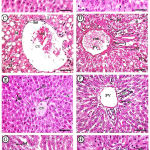 |
Figure 1: Photomicrographs of maternal liver displayed A&B (control group).
|
Showing normal histological architecture of the liver including central vein (CV), blood sinusoid (S), hepatocytes (hc), Kupffer’s cells (k), also showing normal portal venule (PV), bile ductule (BD), and hepatic arteriole (Ar). C&D (Cd group): showing; marked congestion (con) and dilatation of both central and portal, congestion and dilatation of sinusoids (ii), hepatocytes showing marked fatty (fd) and hydropic (hd) degenerations, some hepatocytes showing karyolized (kl) and pyknotic nuclei (py); also, here was marked increase in the inflammatory cells (I). E&F (Zingiber group): showing nearly normal architecture (CV= central vein, S= blood sinusoid, hc= hepatocytes, k= Kupffer’s cells, PV= portal venule, BD= bile ductule, Ar= hepatic arteriole). G&H (Cd+Zingiber group): showing slide congestion (con) of portal vein, dilatation of sinusoids (ii), less marked hydropic degeneration (hd), some inflammatory infilterates (I), some hepatocytes showed karyolized nuclei (kl). H & E x 400 (Scale bar = 500 µm).
Histological Changes of the Fetal liver of Different Groups
Microscopic examination of fetal liver obtained from both control and Zingiber officinale treated groups revealed the normal histological features.
In the fetuses from Cd-treated group, the microscopic examination showed loss of normal hepatic architecture at that age in both centrilobular and periportal areas, dilatation and congestion of the central vein, congestion of sinusoids in both centrilobular and periportal areas, less marked inflammatory cells in both centrilobular and periportal areas, also, congestion of portal venule.
In the fetuses from Cd+Zingiber officinale treated group, the microscopic examination showed a nearly normal picture where the hepatocytes were arranged in the form of cords radiating from the central veins, the hepatic cords were separated by blood sinusoids, which were less marked congested in both centrilobular and periportal areas, congestion of portal venule, increase in inflammatory infiltrates in both centrilobular and periportal areas (Figure 2).
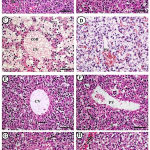 |
Figure 2: Photomicrographs of fetal liver displayed A&B (control group): A&B (control group).
|
Showing normal histological architecture of the fetal liver including central vein (CV), blood sinusoid (S), hepatocytes (hc), some inflammatory cells (I). C&D (Cd group): showing loss of normal architecture of liver at that age in both centrilobular and periportal areas, congestion (con) and dilatation of both central (CV) and portal vein (PV). E&F (Zingiber group): showing nearly normal architecture (CV= central vein, PV= portal vein). G&H (Cd+Zingiber group): showing retaining to some extent normal liver archticture, slight congestion (con) of portal vein, less marked congestion of sinsoids (S). H & E x 400 (Scale bar = 500 µm).
Histological Changes of the Maternal Kidney of Different Groups
In both control and Zingiber officinale treated groups, microscopic examination of the kidney showed the normal histological appearance and structure.
In Cd-treated group, microscopic examination of the kidney showed, degeneration in glomeruli, hydropic degeneration of the cytoplasm and deterioration of the nuclei of the lining cells of the proximal and distal convoluted tubules, multiple areas of haemorrhage in between the tubules, some glomeruli showing widening of urinary space, also there was a marked increase in the inflammatory cellular infiltrate.
In Cd+Zingiber officinale treated group, microscopic examination showed less marked histopathological changes in the form of widening of urinary space, and hydropic degeneration of the cytoplasm and deterioration of the nuclei of the lining cells of the proximal and distal convoluted tubules (Figure 3).
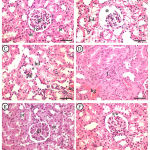 |
Figure 3: Photomicrographs of maternal kidney displayed A&B (control group).
|
Showing a normal histological architecture (gl= glomerulus, pt= proximal convoluted tubule, dt= distal convoluted tubule). C&D (Cd group): showing degenerated glomerulus (dg), areas of hemorrhage (hg) is detected between the tubules, some glomeruli showing widening of urinary space (*) and increased inflammatory cellular infiltrate (I). E&F (Cd+Zingiber group): showing less marked widening of urinary space (**), less marked hydropic degeneration of the cytoplasm and deterioration of the nuclei of the lining cells of the proximal (pt) and distal convoluted tubules (dt). H & E x 400 (Scale bar = 500 µm).
Histological Changes of the Fetal Kidney of Different Groups
In the fetuses revealed from both control and Zingiber officinale treated groups, the microscopic examination of the cortical region of the kidney revealed the normal histological features at that age.
In the fetuses revealed from Cd-treated group, the microscopic examination of kidney showed marked histopathological changes in the form of degeneration of some golmerului, enlarged urinary space, areas of vascular congestion, and increased inflammatory cellular infiltrate.
In the fetuses revealed from Cd+Zingiber officinale treated group, the microscopic examination of kidney showed less damaging features with return back towards the normal structure; only some areas of congestion (Figure 4).
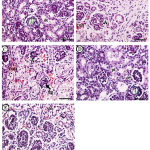 |
Figure 4: Photomicrographs of fetal kidney displayed A (control group).
|
Showing normal histological architecture of the cortical region of kidney, containing a renal corpuscle that consists of Bowman’s capsule enclosing the glomerulus (gl), also, portions of proximal (pt) and distal (dt) convoluted tubules. B&C (Cd group): showing degeneration of some golmerului (dg), iccrease in the urinary space (*) vascular congestion (con), and inflammatory cellular infiltrate (I). D&E (Cd+ Zingiber group): showing, only some areas of congestion (con). H & E x 400 (Scale bar = 500 µm).
Discussion
The rationale of this study is a growing research suggested that maternal exposure to Cadmium poses a risk to women’s health as well as to fetal health and development.33 Results showed that Cd administration to pregnant resulted in adverse effects in mothers and their fetuses. In accordance, it has been stated that in pregnant, Cd gut absorption is increased, causing accumulation of Cd in target tissues as liver and kidney.34
Livers of Cd-treated mother rats showed marked histopathological changes were detected. These results agreed with studies of Ige et al35 and Mahran et al,36 that reported similar changes in liver of Cd-treated rats including an indistinct trabecular structure, necrosis of cells, vacuolar degeneration and mononuclear cell infiltrations. It was reported that these changes are due to Cd toxic effects on hepatocytes since the liver is one of the target organs after Cd chronic exposure, that resulted in structural damage which was accompanied by an increase in hepatic enzymes levels after Cd exposure. In explanation of the morphological hepatic changes, some studies have obviously proved Cd ability to induce oxidative stress as demonstrated by lipid peroxidation, which leads to ROS production, and decline activities of hepatic superoxide dismutase.37
Livers of maternally Cd-treated fetuses showed loss of normal architecture of liver at that age in both centrilobular and periportal areas, dilatation and congestion of the central vein, congestion of portal venule, congestion of sinusoids, less marked inflammatory cells. In agreement to our results, different studies reported that Cd exposure encouraged oxidative impairment in hepatic cells.36
In this study, kidneys of Cd-treated mothers presented many histopathological changes. In accordance, previous studies stated that the kidney is known as a serious target organ of Cd toxicity, where Cd injuries the kidney by escape of essential ions and low-molecular weight proteins into urine, with development to kidney failure.37 This effect is irreversible, and studies reported that the danger occurs also at lower levels of exposure.38 In addition, other studies reported degeneration of renal tubules, epithelial cells hypertrophy, glomeruli dilation and massive local haemorrhage of the kidney tissues of Cd-treated rats.39 The mechanism of Cd-induced renal injury is related to increase in oxidative status, where increased ROS production may be encouraged by the interaction of Cd with mitochondrial structure; resulting in cell necrosis and apoptosis.40
Maternally Cd-treated foetuses, current results showed marked histopathological changes in the kidneys. Some studies have examined the renal effects of maternal exposure with Cd in rats during pregnancy on renal function of the offspring; have been reported a dangerous risk for renal function of their offspring.41
Previous surveys focused on the use of free radical scavengers including minerals (selenium and zinc), vitamins (C and E) and carotenoids that used in management of cellular damage and oxidative stress-mediated diseases caused by Cd exposure, which supports the hypothesis that ROS show a crucial role in Cd toxicity.42-44
In the present study, it was showed that Cd administration to pregnant rats resulted in decreased mean values of fetal growth parameters. In accordance, previous studies about the effects of Cd on embryos have found decreased fetal number and fetal death.45 Also, these studies reported that neonates, may have retarded growth even if born without any apparent disabilities.46 Furthermore, current observations were consistent with studies implicating Cd as having toxic effects on neonatal growth, which was inversely correlated with Cd administration.47 Moreover, studies reported that Cd exposure due to maternal smoking is associated with an increase in congenital malformations and lower birth weight48 and spontaneous abortion.49
For explanation of this fetal toxicity, especially fetal growth restriction, many studies have showed that Cd administered to pregnant animals crosses to the fetus and accumulates in the placenta in high concentrations, and that Cd placental levels were inversely correlated to offspring birth weight; this leads to the suggestion that Cd target the placenta during pregnancy; this accumulation could lead to impaired placental function, thus decreasing nutrients transfer to the fetus, which are crucial for life maintenance and fetal development.50,51
The use of Zingiber officinale during pregnancy is largely realized by its anti-emetic action, where one of the most popular uses of Zingiber officinale is to relief the symptoms of vomiting and nausea accompanying pregnancy in humans.52 The chief ingredients of Zingiber officinale include oleoresin (gingerols and shogaols), volatile oil and phenolic derivatives (zingerone), which are chief antioxidant compounds in Zingiber officinale,53 that depressed lipid peroxidation significantly by preserving the actions of the antioxidant enzymes as glutathione peroxides, catalase and superoxide dismutase.54,55 Hence, there is an imperative for researchers to determine whether Zingiber officinale is useful as natural antioxidant to relief any adverse affects of Cd toxicity on fetal development, which will be a respectable choice as it is used as a food additive. Moreover, the use of ginger during pregnancy does not increase the risk for any of the following pregnancy outcomes: stillbirth/perinatal death, low birth weight and preterm birth.56,57
The current study confirmed the ameliorative activity of Zingiber officinale against Cd toxic effects where neither teratogenic nor embryotoxic effects were observed. This agrees with a previous study in rats, where it was concluded that Zingiber officinale is effective therapeutically against Cd toxicity.58 In accordance, a reproduction study, ginger tea was given during organogenesis (days 6-15) in similar doses like humans. No teratogenicity or maternal toxicity was seen. Furthermore, living female fetuses had advanced skeletal growth and heavier than controls.59 Other study reported that ethanol extract of Zingiber officinale in doses up to 1000 mg/kg/day during organogenesis showed no treatment-related adverse effects, teratogenicity or embryo toxicity were detected in the pregnant mothers or the offspring compared to a controls.60
In Zingiber officinale co-treated rats, an improvement in the Cd-damage of the liver and kidney of mothers and fetuses was observed. The present results agreed with published data by Egwurugwu et al.58 and Gehan and Ayman,61 who reported that Zingiber officinale showed an antagonistic action on Cd toxicity.
Conclusion
Results from this study have demonstrated that Zingiber officinale through its antioxidant activity might be considered beneficial against toxic effects of Cd exposure during pregnancy.
Conflict of Interest
There is no conflict of interest.
References
- Vahter M, Berglund M, Akesson A, Liden C. Metals and women’s health. Environmental research. 2002;88:145-155.
CrossRef - Suwazono Y, Kobayashi E, Okubo Y, Nogawa K, Kido T, Nakagawa H. Renal effects of cadmium exposure in cadmium nonpolluted areas in Japan. Environmental research. 2000;84:44-55.
CrossRef - Satarug S, Garrett S.H, Sens M.A, Sens D.A. Cadmium, environmental exposure, and health outcomes. Ciencia & saude coletiva. 2011;16:2587-2602.
CrossRef - Nwokocha C.R, Nwokocha M.I, Owu D.U, et al. Estimation of absorbed cadmium in tissues of male and female albino rats through different routes of administration. Nigerian journal of physiological sciences :official publication of the Physiological Society of Nigeria. 2011;26:97-101.
- Rahimzadeh R.M, Rahimzadeh R.M, Kazemi S, Moghadamnia A.A. Cadmium toxicity and treatment: An update. Caspian J Intern Med. 2017;8:135-145.
- El-Sharkawy S.L, Abbas N.F, EL-Hefnawy N.G. Prognostic Markers in Prostatic Carcinoma. Methods of Cancer Diagnosis, Therapy and Prognosis: General Methods and Overviews, Lung Carcinoma and Prostate Carcinoma. 2009;2:465.
CrossRef - Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicology and applied pharmacology. 2009;238:201-208.
CrossRef - Ognjanovic B.I, Markovic S.D, Ethordevic N.Z, Trbojevic I.S, Stajn A.S, Saicic Z.S. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q(10) and vitamin E. Reproductive toxicology (Elmsford, N.Y.). 2010;29:191-197.
CrossRef - Godt J, Scheidig F, Grosse-Siestrup C, et al. The toxicity of cadmium and resulting hazards for human health. Journal of occupational medicine and toxicology (London, England). 2006;1:22.
CrossRef - Newairy A.A, El-Sharaky A.S, Badreldeen M.M, Eweda S.M, Sheweita S.A. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology. 2007;242:23-30.
CrossRef - Asagba S.O, Eriyamremu G.E, Adaikpoh M.A, Ezeoma A. Levels of lipid peroxidation, superoxide dismutase, and Na+/K+ ATPase in some tissues of rats exposed to a Nigerian-like diet and cadmium. Biol Trace Elem Res. 2004;100:75-86.
CrossRef - Thijssen S, Maringwa J, Faes C, Lambrichts I, Van Kerkhove E. Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicology. 2007;229:145-156.
CrossRef - Akesson A, Berglund M, Schutz A, Bjellerup P, Bremme K, Vahter M. Cadmium exposure in pregnancy and lactation in relation to iron status. American journal of public health. 2002;92:284-287.
CrossRef - Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environmental research. 2007;104:85-95.
CrossRef - Nishijo M, Nakagawa H, Honda R, et al. Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup Environ Med. 2002;59:394-396.discussion 397.
- Thompson J, Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reproductive toxicology (Elmsford, N.Y.). 2008;25:304-315.
CrossRef - Guan H, Piao F.Y, Li X.W, Li Q.J, Xu L, Yokoyama K. Maternal and fetal exposure to four carcinogenic environmental metals. Biomedical and environmental sciences : BES. 2010;23:458-465.
CrossRef - Stohs S.J, Bagchi D, Hassoun E, Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. Journal of environmental pathology, toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer. 2001;20:77-88.
- Ikediobi C.O, Badisa V.L, Ayuk-Takem L.T, Latinwo L.M, West J. Response of antioxidant enzymes and redox metabolites to cadmium-induced oxidative stress in CRL-1439 normal rat liver cells. International journal of molecular medicine. 2004;14:87-92.
CrossRef - Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369-382.
CrossRef - Masso E.L, Corredor L, Antonio M.T. Oxidative damage in liver after perinatal intoxication with lead and/or cadmium. J Trace Elem. Med .Biol. 2007;21:210-216.
CrossRef - Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reproductive toxicology (Elmsford, N.Y.). 2007;24:31-41.
CrossRef - Dugasani S, Pichika M.R, Nadarajah V.D, Balijepalli M.K, Tandra S, Korlakunta J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515-520.
CrossRef - Gabr S.A, Alghadir A.H, Ghoniem G.A. Biological activities of ginger against cadmium-induced renal toxicity. Saudi Journal of Biological Sciences. 2017.
CrossRef - Farag A.G.A, Elhalwagy M.E.A, Farid H.E.A. Effect of ginger supplementation on developmental toxicity induced by fenitrothion insecticide and/or lead in albino rats. Pesticide Biochemistry and Physiology. 2010;97:267-274.
CrossRef - Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683-690.
CrossRef - Ahmed R.S, Suke S.G, Seth V, Chakraborti A, Tripathi A.K, Banerjee B.D. Protective effects of dietary ginger (Zingiber officinales Rosc.) on lindane-induced oxidative stress in rats. Phytotherapy research : PTR. 2008;22:902-906.
- Rani A, Kumar A, Lal A, Pant M. Cellular mechanisms of cadmium-induced toxicity: a review. International journal of environmental health research. 2014;24:378-399.
CrossRef - Kamtchouing P, Mbongue Fandio G.Y, Dimo T, Jatsa H.B. Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian J Androl. 2002;4:299-301.
- Chemek M, Boughammoura S, Mimouna S.B, Chouchene L, Banni M, Messaoudi I. Changes of the mRNA expression pattern of Zn transporters: a probable mechanism for cadmium retention and zinc redistribution in the suckling rat tissues. Biol Trace Elem Res. 2015;165:173-182.
CrossRef - Chemek M, Mimouna S.B, Boughammoura S, Delbes G, Messaoudi I. Protective role of zinc against the toxicity induced by exposure to cadmium during gestation and lactation on testis development. Reproductive toxicology (Elmsford, N.Y.). 2016;63:151-160.
CrossRef - Baranski B. Effect of maternal cadmium exposure on postnatal development and tissue cadmium, copper and zinc concentrations in rats. Archives of toxicology. 1986;58:255-260.
CrossRef - Massanyi P, Lukac N, Uhrin V, et al. Female reproductive toxicology of cadmium. Acta biologica Hungarica. 2007;58:287-299.
CrossRef - Zalups R.K, Koropatnick D.J. Molecular Biology and Toxicology of Metals: Taylor & Francis. 2000.
- Ige S.F, Salawu E.O, Olaleye S.B, Adeeyo O.A, Badmus J, Adeleke A.A. Onion (Allium cepa) extract prevents cadmium induced renal dysfunction. Indian J Nephrol. 2009;19:140-144.
CrossRef - Mahran A.A.H,Osman H.E. Protective Effect of Zinc (Zn) on the Histology and Histochemistry of Liver and Kidney of Albino Rat Treated with Cadmium. Journal of Cytology & Histology. 2011;02:2-9.
CrossRef - Tarasub N, Junseecha T, Tarasub C, Ayutthaya N.W.D. Protective Effects of Curcumin, Vitamin C, or their Combination on Cadmium-Induced Hepatotoxicity. Journal of basic and clinical pharmacy. 2012;3:273-281.
CrossRef - Satarug S, Haswell-Elkins M.R, Moore M.R. Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br J Nutr. 2000;84:791-802.
- Obianime A.W,Roberts I.I. Antioxidants, cadmium-induced toxicity, serum biochemical and the histological abnormalities of the kidney and testes of the male Wistar rats. Nigerian journal of physiological sciences :official publication of the Physiological Society of Nigeria. 2009;24:177-185.
- Tang W, Shaikh Z.A. Renal cortical mitochondrial dysfunction upon cadmium metallothionein administration to Sprague-Dawley rats. Journal of toxicology and environmental health. Part A. 2001;63:221-235.
CrossRef - Jacquillet G, Barbier O, Rubera I, et al. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am J Physiol Renal Physiol. 2007;293:F1450-1460.
CrossRef - Fouad A.A, Qureshi H.A, Yacoubi M.T, Al-Melhim W.N. Protective role of carnosine in mice with cadmium-induced acute hepatotoxicity. Food .Chem. Toxicol. 2009;47:2863-2870.
CrossRef - Amara S, Abdelmelek H, Garrel C, et al. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. The Journal of reproduction and development. 2008;54:129-134.
CrossRef - Karabulut-Bulan O, Bolkent S, Yanardag R, Bilgin-Sokmen B. The role of vitamin C, vitamin E, and selenium on cadmium-induced renal toxicity of rats. Drug and chemical toxicology. 2008;31:413-426.
CrossRef - Lin C.M, Doyle P, Wang D, Hwang Y.H, Chen P.C. Does prenatal cadmium exposure affect fetal and child growth? Occup Environ Med. 2011;68:641-646.
CrossRef - Blum J.L, Xiong J.Q, Hoffman C, Zelikoff J.T. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicological sciences : an official journal of the Society of Toxicology. 2012;126:478-486.
CrossRef - Källén K. Maternal smoking during pregnancy and infant head circumference at birth. Early Human Development. 2000;58:197-204.
CrossRef - Leary S, Smith D.G, Ness A. Smoking during pregnancy and components of stature in offspring. American journal of human biology : the official journal of the Human Biology Council. 2006;18:502-512.
CrossRef - Apostoli P, Catalani S. Metal ions affecting reproduction and development. Metal ions in life sciences. 2011;8:263-303.
- Osada H, Watanabe Y, Nishimura Y, Yukawa M, Seki K, Sekiya S. Profile of trace element concentrations in the feto-placental unit in relation to fetal growth. Acta obstetricia et gynecologica Scandinavica. 2002;81:931-937.
CrossRef - Henson M.C, Chedrese P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood). 2004;229:383-392.
CrossRef - Gilani A.H, Rahman A.U. Trends in ethnopharmocology. J Ethnopharmacol. 2005;100:43-49.
CrossRef - Masuda Y, Kikuzaki H, Hisamoto M, Nakatani N. Antioxidant properties of gingerol related compounds from ginger. BioFactors. 2004;21:293-296.
CrossRef - Zancan K.C, Marques M.O.M, Petenate A.J, Meireles M.A.A. Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and co-solvents: a study of the antioxidant action of the extracts. The Journal of Supercritical Fluids. 2002;24:57-76.
CrossRef - Topic B, Tani E, Tsiakitzis K, et al. Enhanced maze performance and reduced oxidative stress by combined extracts of zingiber officinale and ginkgo biloba in the aged rat. Neurobiol Aging. 2002;23:135-143.
CrossRef - Shawahna R, Taha A. Which potential harms and benefits of using ginger in the management of nausea and vomiting of pregnancy should be addressed? a consensual study among pregnant women and gynecologists. BMC Complement Altern Med. 2017;17:204.
CrossRef - Heitmann K, Nordeng H, Holst L. Safety of ginger use in pregnancy: results from a large population-based cohort study. European journal of clinical pharmacology. 2013;69:269-277.
CrossRef - Egwurugwu J, Ufearo C, Abanobi O, et al. Effects of ginger (Zingiber officinale) on cadmium toxicity. African Journal of Biotechnology. 2007;6.
- Wilkinson J.M. Effect of ginger tea on the fetal development of Sprague-Dawley rats. Reproductive toxicology (Elmsford, N.Y.). 2000;14:507-512.
CrossRef - Weidner M.S, Sigwart K. Investigation of the teratogenic potential of a Zingiber officinale extract in the rat. Reproductive Toxicology. 2000;15:75-80.
CrossRef - Gehan A, Ayman Y. Cadmium-ginger two way antagonistitc relationship. Arab J Biochem. 2010;13:115-124.







