Manuscript accepted on :30-Aug-2018
Published online on: 18-09-2018
Plagiarism Check: Yes
Reviewed by: M. Mohan Varma
Second Review by: Abhinav Kanwal
Final Approval by: Dr. H Fai Poon
A. Umamaheswari1, K. Bhuvaneswari1 and R. Senthilkumar2
1Department of Pharmacology, PSG Institute of Medical Sciences and Research, The Tamilnadu Dr MGR Medical University, Coimbatore, India.
2Department of Endocrinology, PSG Institute of Medical Sciences and Research, The Tamilnadu Dr MGR Medical University, Coimbatore, India.
Corresponding Author E-mail: dr.uma.pharmac@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1535
Abstract
Insulin resistance and endothelial dysfunction which shares multiple signaling pathways like hyperinsulinemia, glucotoxicity and inflammation in type 2 Diabetes Mellitus (DM) leads to several micro and macrovascular complications. Studies have shown the anti-inflammatory effects of certain oral hypoglycemic agents which will be helpful in preventing the impact of diabetes related complications. The study aimed to compare the anti-inflammatory effects of Sitagliptin and Acarbose in combination with Metformin and Sulfonylurea in Type 2DM patients by using Anti-inflammatory markers Interleukin-6 (IL6), high sensitive C-reactive protein (hsCRP) and also to compare the clinical outcome between these two groups by using the parameters Fasting blood sugar (FBS), Post prandial blood sugar (PPBS), hemoglobin A1c (HbA1C), Plasma Insulin. In this open labeled prospective parallel group clinical study 30 type 2 diabetes patients on Metformin and Sulfonylurea combination, with HbA1c value ≥7.5 were recruited in tertiary care hospital and divided into two groups based on their HbA1C levels and were added on either Acarbose or Sitagliptin along with Metformin Sulfonylurea combinations and were followed for 3 months. Parameters like FBS, PPBS, HbA1c, Plasma Insulin hsCRP, IL-6were measured before and after the study. In the study the mean value of FBS, PPBS, HbA1c, Plasma Insulin, Insulin Resistance, hsCRP were reduced in both Sitagliptin and Acarbose group, which were similar to the results of previous studies except IL6 which got reduced in Sitagliptin group but increased in Acarbose group. The study had showed the synergism of Sitagliptin with Metform in Sulfonylurea combinationin reducing inflammation however; still long term studies are required to confirm their anti-inflammatory effects.
Keywords
Acarbose; Cardiovascular Risk; High Sensitive C-Reactive Protein; Interleukin-6; Sitagliptin
Download this article as:| Copy the following to cite this article: Umamaheswari A, Bhuvaneswari K, Senthilkumar R. To Compare the Anti-Inflammatory Effect of Oral Hypoglycemic Drugs in Type 2 Diabetes Mellitus. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Umamaheswari A, Bhuvaneswari K, Senthilkumar R. To Compare the Anti-Inflammatory Effect of Oral Hypoglycemic Drugs in Type 2 Diabetes Mellitus. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22741 |
Introduction
Diabetes mellitus is one of the non communicable diseases which have become a major global health problem.1 This is mainly due to insulin resistance or decreased production of insulin. Insulin resistance, one of the key factor common to obesity and type 2 DM, is associated with endothelial dysfunction and increases the cardiovascular risk2 by sharing multiple signaling pathways which include hyperinsulinemia, glucotoxicity, lipotoxicity and inflammation.2
In 2010, it was found that 285 million people around world have diabetes, of which 80% are from developed countries. The prevalence of diabetes is expected to reach about 438 million by 2030.1,3
Type 2 DM is characterized by improper secretion of insulin, resistance to insulin, increased glucose production in liver, and abnormal lipid metabolism. Most studies say that insulin resistance occurs before the insulin secretory defect and type 2 diabetes develops only when insulin secretion become less. In the earlier stages of diabetes, glucose tolerance is found to be normal, because pancreatic beta cell gives a compensatory increase in insulin output which further leads to insulin resistance after a certain period of time leading to a state called Impaired Glucose Tolerance (IGT), where there will be high postprandial glucose level. Later reduction in secretion of Insulin and high hepatic glucose formation leads to overt diabetes with increasing blood glucose values, indicating the failure of beta cells. 4
The obesity in diabetes increases the levels of fat cell products like, nonesterified free fatty acids, retinol-binding protein 4, leptin, Tumor Necrosis Factor (TNF)-α, resistin, adiponectin and free fatty acids (FFA) in circulation. Adipokines modulate the sensitivity of insulin plus increase in FFA production, causes resistance of insulin in hepatic and skeletal muscle. Products of adipocyte ground a state of inflammation and in turn they involve in the pathogenesis of insulin resistance and endothelial dysfunction.5-11
Diabetes -Inflammation – Cardiovascular risk
The hypotheses propose that hyperglycemia and insulin resistance increases FFA and forms diacylglycerol leading to stimulation of protein kinase C (PKC). PKC modifies the gene transcription of endothelial cells.12Endothelial dysfunction presents in patients with insulin resistance in stages prior to the development of impaired glucose tolerance (IGT) and type 2 DM. This suggests that insulin resistance is key factor and endothelial dysfunction is not simply a result of hyperglycemia.
Rise in FFA stimulates toll like receptor (TLR) and Nuclear Factor-kB (NF-kB) and subsequently promotes the genes of inflammation like TNF-αand Interleukin-6 (IL-6), followed by PKC activation which decreases insulin sensitivity. Impaired Insulin sensitivity in endothelium of the blood vessels promotes Reactive oxygen species (ROS) formation, FFA oxidation, which subsequently stimulates PKC activation leading to advanced glycated end products (AGE)synthesis, and down-regulates Prostacyclin(PGI2). Finally endothelial nitric oxide synthatase(eNOS) activity is impaired leading to endothelial dysfunction.12-14
Obese diabetic patients treated with Metformin have proven to lower the levels of hsCRP, expression of TNF-α and TLR 2/4.15 Previous studies proved that Sitagliptin apart from stimulating the Adenosine Mono phosphate (AMPK)phosporylation, it also inhibit the activation of Mitogen activated protein kinase (MAPK) including p38 and Extracellular signal regulated kinase(ERK)and arrest the progression of atherosclerosis leading to decreases in adhesion molecules and inflammatory cytokines like hsCRP, IL-6 and FFA levels after 12 weeks of therapy.16-19 It concludes the anti-inflammatory action of Sitagliptin and its effect in inhibition of atherosclerosis.20 The results from the previous studies revealed, that the Acarbose treatment showed hopeful benefits in cardiovascular disease by decreasing the proinflammatory transcription factor (NFκ B) activity and hsCRP levels21-24
Based on this background information, the study targeted the oral hypoglycemic agents like Acarbose and Sitagliptin to look for their anti-inflammatory action.Hence the study aimed to compare the anti-inflammatory effects of Metformin sulfonylurea and Sitagliptin combination with Metformin Sulfonylurea and Acarbosecombination in Type2DM patients by using Anti-inflammatory markers (IL6, hsCRP) and also to compare the clinical outcome between these two groups by using the following parameters likeFBS, PPBS, HbA1C, and Plasma Insulin.
Materials and Methods
This study was an open labeled prospective parallel groupclinical study. After getting approval from the Institute Human Ethics Committee (IHEC) (proposal no: 12/282), 30 type 2 diabetic patients (convenient sample) attending Endocrinology and Medicine OPD in a Tertiary care hospital Coimbatore were recruited. We included all type 2 diabetic patients in an age group of 25-65 years, who were on conventional treatment of Metformin Sulfonylurea combination with HbA1C level > 7.5%.Patients with diabetes other than type 2, history of cardiac, renal and cerebrovascular diseases, patients on treatment with statins and steroids, history of altered liver function and pancreatitis, known history of alcohol intake and smoking were excluded from the study. The details of the study were explained to each participant individually before getting the informed consent. Basic data’s like name, age and height, weight were recorded and comorbid conditions were noted.
Then the clinical examination and the base line investigations (Fasting blood glucose, postprandial blood glucose, HbA1C, hsCRP, IL6, Plasma Insulin) were done before starting the treatment. Later those 30 participants were divided into two groups based on their HbA1C values with 15 patients in each group. One group was on Sitagliptin, and the other group was on Acarbose treatment along with their conventional Metformin and Sulphonylurea combination. Sitagliptin was started on 50mg OD and then titrated to 100mg according to the patient’s blood glucose level by the physician. In the same way, Acarbose was started on 25mg BD initially and later it was titrated to 50mg BD or 25mg TDS according to the patient’s blood glucose level .The study participants were advised to come for review after two weeks for titration of the drug dosage and followed for 3 months. In between the 3 months of study period, the participant‘s FBS, PPBS levels were noted in the case file.
Finally at the end of three months the clinical examination and the investigations (FBS, PPBS, HbA1C, IL6, hsCRP and Plasma insulin) were done again and the study was completed.
The results of the parameters were analyzed in the hospital laboratory for FBS, PPBS, HbA1c, hsCRP, Plasma Insulin. By using the formula of Homa Index, HOMA-IR = [Fasting glucose (nmol/L) * insulin (µU/mL)/22.5]Insulin Resistance for all the 30 patients were calculated. The Interleukin-6 (Krishgen Biosystems) was estimated in the Pharmacology laboratory by enzyme immunoassay which wasprogrammed for quantification of human interleukin-6 and analyzed.
Statistical Analysis
Data’s were analyzed using SPSS soft ware version 19.0. Values between the two groups were interpreted using independent sample t-test and before and after values within the same groups with paired t test.
Results
Among the total number of 30 patients 77% of study population were under 55 years of age and 23% were between 55 to 65 years of age. In that 57% were male and 43% were female patients.
Regarding the duration, 50% were in between 0-5 years , 37% of patients were between 6-10 yrs, and 13% of patients were beyond 11years of duration of DM .This mainly implied the chronicity and progression of disease pattern in those study participants.
Results of BMI in Acarbose and Sitagliptin groups showed no statistical significance even though there was greater reduction in Sitagliptin group in which mean value decreased from 27.02 + 4.64 SD to 26.32 + 4.81 SD ( p value 0.033) (Table 1). End values of FBS in the study showed reduction in mean value in both Acarbose and Sitagliptin group ranging from 183.71 +44.99 SD to 142.92 +33.60 SD, 178.07 + 46.73 SD to 138.84 +25.13 SD with p value 0.007, 0.020 respectively(Table 1). Regarding PPBS it showed reduction in mean value in both Acarbose and Sitagliptin group ranging from 272.35 + 64.08 SD to 204.85 + 57.97 SD , 263.92 + 101.60 SD to 194.23 + 44.50 SD with p value 0.006, 0.025 respectively (Table 1).
Results of HbA1c in the study showed statistical reduction in mean value in both Acarbose and Sitagliptin group ranging from 10.01 + 1.51 SD to 8.17 + 1.28 SD , 9.69 +1.53 SD to 7.75 + 1.32 SD (p value of 0.001, 0.004) respectively(Table 1). Results of Plasma Insulin in the study showed reduction in mean value in both Acarbose and Sitagliptin group ranging from 18.71 + 16.42 SD to 14.18 + 6.71 SD , 27.72 +25.98 to 22.58 + 17.68 SD respectively. This was of no statistical significance because of p value = 0.327, 0.324 respectively (Table 1). End values of IR in this study showed reduction in mean value in both Acarbose and Sitagliptin group ranging from 9.00 + 8.38 SD to 5.12 + 3.14 SD , 11.49 +9.42 SD to 7.63 + 5.78 SD respectively (p value = 0.100 , 0.101) (Table 1) but no statistical significance.
Results of hsCRP in this study showed reduction in mean value in both Acarbose and Sitagliptin group ranging from 0.58 + 0.93 SD to 0.30 + 0.30 SD, 0.45 + 0.43 SD to 0.41 + 0.39 SD respectively. This was of no statistical significance (p value = 0.265, 0.671 respectively) (Table 1).
Results of IL-6 in this study showed reduction in mean value of Sitagliptin group ranging from 136.32 + 241.81 SD to 57.679 + 78.55 SD (p value =0.314 ) but in case of Acarbose group there was slight increase in IL-6 levels with the mean value ranging from 120.01 + 203.3 SD to 174.08 + 492.1 SD .(p value=0.591) (Table 1).
At the end of three months there was no significant difference seen in the reduction of FBS (p value = 0.725), PPBS (p value = 0.600), HbA1C (p value =0.403), Plasma Insulin (p value =0.110), Insulin Resistance (p value =0.170), hsCRP (p value =0.451) IL-6 (p value = 0.408) on comparing the after values of Acarbose and Sitagliptin group.
Discussion
It is a well known fact that, the risk of cardiovascular disorder is high in both type I and type II Diabetes Mellitus .In case of type I DM risk of CVD arise after some decades but in type II DM, it sets very early in the asymptomatic period of hyperglycemia and even by the time of diagnosis these patients are at potential risk. This is mainly due to the Insulin Resistance, which emerges at an early stage of type II DM. Obesity in diabetes along with inflammation leads to endothelial dysfunction and IR which then subsequently causes platelet aggregation, and all this process collectively leads to atherogenesis and cardiovascular disease ultimately. It has been proven that increase in the inflammatory markers would predict the risk of cardiovascular complications at the earliest.
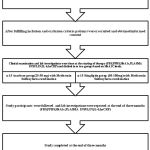 |
Figure 1: Methodology of anti-inflammatory effect of oral hypoglycemic drugs.
|
Previous studies states that drugs like Metformin, Sitagliptin and Acarbose effectively reduces the inflammatory markers TNF-α, IL-6, hsCRP and NF-kB levels in diabetes patients and facilitate the prevention of endothelial dysfunction and atherosclerosis.15,20,24,25
 |
Figure 2: hs CRP status in Acarbose and Sitagliptin groups at 1st = month and at the end of 3rd month.
|
In this study the results of BMI showed greater reduction in the Sitagliptin group with mean value ranging from 27.02 +4.64 SD to 26.32 + 4.81SD, p value = 0.033 compared to the Acarbose group. These were similar to the results of previous studies.20
 |
Figure 3: IL-6 status in Acarbose and Sitagliptin groups at 1st = month and at the end of 3rd month.
|
Regarding the mean values of FBS, PPBS, HbA1c, Plasma Insulin, Insulin Resistance, both Sitagliptin and Acarbose combination groups showed good reduction in their mean value, which were again similar to the results of previous studies.20,22,27
Then mean levels of hsCRP at the starting of study in Acarbose and Sitagliptin groups were found to be 0.58 +0.93 SD and 0.45 + 0.43 SD respectively. At the end of the three months levels of hsCRP were reduced in both Acarbose and Sitagliptin groups to the mean value of 0.30+0.3 SD (p value= 0.265) and 0.41 + 0.39 SD (p value= 0.671) (Table 1) respectively. Though there was greater reduction in mean values of hsCRP levels which were consistent with the results of previous studies [20, 25, 26] these results were not statistically significant. When the values of hsCRP were compared between Acarbose and Sitagliptin groups, at the end of the three months there were no statistical significant reduction in hsCRP values (p value=0.451).
Next inflammatory marker analyzed was the IL-6 levels. The mean values in the starting of treatment were 120.01 + 203.3 SD in Acarbose group and 136.32 + 241.81 SD in Sitagliptin group. At the end of the three months the mean values of IL-6 in Acarbose group was found to be increased to the mean value of 174.08 + 492.1 SD but in Sitagliptin group it was found to be reduced to 57.69 + 78.55 SD.When the before and after values of Sitagliptin group was analyzed, it showed reduction in the mean values of IL-6 levels at the end of the three months. But there was no statistical significance (p value =0.314) (Table 1).
Table 1: Mean values of the test parameters.
| Week 0 (baseline) mean ± SD | Week 12 mean ± SD | Mean difference | P value | |
| BMI | ||||
| Acarbose | 25.20+2.84 | 25.25+2.83 | -.056+.79 | 0.796 |
| Sitagliptin | 27.02+4.64 | 26.32+4.81 | 0.69+1.04 | 0.033 |
| FBS | ||||
| Acarbose | 183.71+44.99 | 142.92+33.60 | 40.78+47.75 | 0.007 |
| Sitagliptin | 178.07+46.73 | 138.84+25.13 | 39.23+52.50 | 0.020 |
| PPBS | ||||
| Acarbose | 272.35+64.08 | 204.85+57.97 | 67.50+76.83 | 0.006 |
| Sitagliptin | 263.92+101.60 | 194.23+44.50 | 69.69+98.3 | 0.025 |
| HbA1c | ||||
| Acarbose | 10.01+1.51 | 8.17+1.28 | 1.83+1.67 | 0.001 |
| Sitagliptin | 9.69+1.53 | 7.75+1.32 | 1.94+1.99 | 0.004 |
| Plasma Insulin | ||||
| Acarbose | 18.71+16.42 | 14.18+6.71 | 4.52+16.60 | 0.327 |
| Sitagliptin | 27.72+25.98 | 22.58+17.68 | 5.13+17.9 | 0.324 |
| Insulin Resistance | ||||
| Acarbose | 9.00+8.38 | 5.12+3.14 | 3.88+8.19 | 0.100 |
| Sitagliptin | 11.49+9.42 | 7.63+5.78 | 3.86+7.84 | 0.101 |
| hsCRP | ||||
| Acarbose | 0.58+0.93 | 0.30+0.3 | 0.27+0.89 | 0.265 |
| Sitagliptin | 0.45+0.43 | 0.41+0.39 | 0.48+0.40 | 0.671 |
| IL6 | ||||
| Acarbose | 120.01+203.3 | 174.08+492.1 | -54.07+36.7 | 0.591 |
| Sitagliptin | 136.32+241.81 | 57.67+78.55 | 78.64+269.97 | 0.314 |
When comparing the after values of IL-6 in Acarbose and Sitagliptin groups, greater reduction was seen in the Sitagliptin group after 3 months of therapy. Thus the findings in the study were consistent with the previous studies which showed the anti- inflammatory effect of Sitagliptin therapy.20 But this is again not statistically significant (p value = 0.408).
Concerning the results of the anti-inflammatory markers in Sitagliptin treated group with metformin sulphonylureas combination showed a definite reduction in the mean values of both hsCRP, IL-6 levels after the three months treatment, but still it was not statistically significant. This may be due to less sample size, and the patients were followed only for 3 months. A long term follow up and a bigger sample size ismandatory to get a statistically significant results.Sitagliptin has proven to have a definite role in reducing chronic inflammation. Based on this study finding, fixed dose combination of Sitagliptin and Metformin might be helpful in preventing the progress of diabetes.
When the results of inflammatory markers in Acarbose group were analyzed and compared with previous studies,22-24 Acarbose also reduced the inflammatory markers moderately in this study. This study showed a greater reduction in the mean value of hsCRP levels but not in IL-6, this might be because of small sample size, poor compliance of patients and the patients were followed only for 3 months. Hence long term follow up and large sample size may be required to see the progression in the reduction of inflammatory markers and the role of these drugs in the insulin resistant states. During the study period no adverse reactions were noted among the study participants.
Conclusion
This study demonstrated the additive anti-inflammatory effect of Sitagliptin, and Acarbose as these groups of drugs are now in trend and commonly been prescribed either as base line therapy or as add on therapy based on the HbA1C values. When such anti inflammatory property is pronounced with Sitagliptin, it can be sure that addition of Sitagliptin with Metformin will be a beneficial combination at the initial therapy to the patients in preventing the progression of diabetic related cardio vascular complications.26
Limitations
In this study the patient sample size was small and the study duration was 3 months which is again one of the limitations to assess the anti-inflammatory effects of these drugs on long term effects. Hence long term follows up and bigger sample size may be mandatory in future to get the statistically significant reduction in the inflammatory markers.
Conflict of Interest
There is no conflict of interest.
Funding Source
Self funding and Post graduate thesis funding by the Institution.
Acknowledgement
We express our sincere thanks to Dr. Saravanan and Dr.SujithKumar (Medicine department, PSGIMSR.) and Dr. Suresh (Endocrinology dept. PSGIMSR) for their help in the execution of this study. We also like to thank the study participants for their cooperation to complete this study.
References
- Roglic G. Editor. World Diabetes Foundation summit for Latin America. Estimates of the global and regional burden of diabetes [Internet]. World Health Organization,Salvador (Brazil) [cited 2014 Sep 15].Available. 2010. from: https://www.worlddiabetesfoundation.org/sites/default/files/WDF_Brazil_Summit_Report_2010. pdf.
- Turco D . S., Gaggini M., Daniele G., Basta G., Folli F., Sicari R., Gastaldelli A. Insulin resistance and endothelial dysfunction: a mutual relationship in cardiometabolic risk. Current pharmaceutical design. 2013;19(13):2420-31.
CrossRef - Unwin N., Whiting D., Gan D., Jacqmain O., Ghyoot G. editors. IDF diabetes atlas. International Diabetes Federation. 2009.
- Fauci A. S., Braunwald E. editors. Harrison’s Principles of Internal Medicine. 17th ed. New York McGraw-Hill. 2008;2275
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87-91.
CrossRef - Maachi M., Pieroni L., Bruckert E., Jardel C., Fellahi S., Hainque B., Capeau J., Bastard J. P. Systemic low-grade inflammation is related to circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. International journal of obesity. 2004;28(8):993-7.
CrossRef - Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annual review of immunology. 1995;13(1):369-98.
CrossRef - Mooney R. A., Senn J., Cameron S., Inamdar N., Boivin L. M., Shang Y., Furlanetto R. W. Suppressors of cytokine signaling-1 and-6 associate with and inhibit the insulin receptor A potential mechanism for cytokine-mediated insulin resistance. Journal of Biological Chemistry. 2001;276(28):25889-93.
CrossRef - Rieusset J., Bouzakri K., Chevillotte E., Ricard N., Jacquet D., Bastard J. P., Laville M., Vidal H. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53(9):2232-41.
CrossRef - Kroder G., Bossenmaier B., Kellerer M., Capp E., Stoyanov B., Mühlhöfer A., Berti L., Horikoshi H., Ullrich A., Häring H. Tumor necrosis factor-alpha-and hyperglycemia-induced insulin resistance. Evidence for different mechanisms and different effects on insulin signaling. Journal of Clinical Investigation. 1996;97(6):1471.
CrossRef - Yudkin J. S., Kumari M., Humphries S. E., Mohamed-Ali V. Inflammation obesity stress and coronary heart disease is interleukin-6 the link. Atherosclerosis. 2000;148(2):209-14.
CrossRef - Du X., Edelstein D., Obici S., Higham N., Zou M. H., Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. Journal of Clinical Investigation. 2006;116(4):1071.
CrossRef - Paneni F., Beckman J. A., Creager M. A., Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. European heart journal. 2013;34(31):2436-43.
CrossRef - Hink U., Li H., Mollnau H., Oelze M., Matheis E., Hartmann M., Skatchkov M., Thaiss F., Stahl R. A., Warnholtz .A, Meinertz T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circulation research. 2001;88(2):e14-22.
- Andrews M., Soto N., Arredondo M. Effect of metformin on the expression of tumor necrosis factor-α, Toll like receptors 2/4 and C reactive protein in obese type-2 diabetic patients. Revistamedica de Chile. 2012;140(11):1377-82.
CrossRef - Choi M. K., Jin Q. R., Ahn S. H., Bae M. A., Song I. S. Sitagliptin attenuates metformin-mediated AMPK phosphorylation through inhibition of organic cation transporters. Xenobiotica. 2010; 40(12):817-25.
CrossRef - Lenski M., Kazakov A., Marx N., Böhm M., Laufs U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. Journal of molecular and cellular cardiology. 2011;51(6):906-18.
CrossRef - Ikeda T., Kumagai E., Iwata S., Yamakawa A. Soluble CD26 dipeptidyl peptidase IV enhances the transcription of IL-6 and TNF-α in THP-1 cells and monocytes. PLoS One. 2013;8(6):e66520.
- Erdogdu Ö., Eriksson L., Xu H., Sjöholm Å., Zhang Q., Nyström T. Exendin-4 protects endothelial cells from lipoapoptosis by PKA, PI 3 K, eNOS, p 38 MAPK and JNK pathways. Journal of molecular endocrinology. 2013;50(2):229-41.
CrossRef - Makdissi A., Ghanim H.,Vora M.,et al. Sitagliptin Exerts an Ant inflammatory Action. J Clin. Endocrinol Metab. 2012;97(9):3333–41.
CrossRef - Hanefeld M., Cagatay M., Petrowitsch T., Neuser D., Petzinna D., Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. European heart journal. 2004;25(1):10-6.
CrossRef - Rudofsky Jr G., Reismann P., Schiekofer S., Petrov D., Eynatten V. M., Humpert P. M., Isermann B., Müller-Hoff C., Thai T. P., Lichtenstein S., Bärtsch U. Reduction of postprandial hyperglycemia in patients with type 2 diabetes reduces NF-κB activation in PBMCs. Hormone and metabolic research. 2004;36(09):630-8.
CrossRef - Tschoepe D. Decreased fibrinogen by treatment with the alpha-glucosidase inhibitor acarbose. Diabetes. 2004;53:189.
- Wang X., Lu J., Pan C. Comparison of serum C-reactive protein level in different glucose tolerance subjects and the change in serum CRP level in IGT subjects with acarbose [abstract 1634]. Chin J Endocrinol. Metab. 2003;19:254-6.
- Rudofsky Jr G., Reismann P., Schiekofer S., Petrov D., Eynatten V. M., Humpert P. M., Isermann B., Müller-Hoff C., Thai T. P., Lichtenstein S., Bärtsch U. Reduction of postprandial hyperglycemia in patients with type 2 diabetes reduces NF-κB activation in PBMCs. Hormone and metabolic research. 2004;36(09):630-8.
CrossRef - Reasner C., Olansky L., Seck T. L., Williams‐Herman D. E., Chen M., Terranella L., Johnson‐Levonas A. O., Kaufman K. D., Goldstein B. J. The effect of initial therapy with the fixed‐dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obesity and Metabolism. 2011;13(7):644-52.
CrossRef







