Manuscript accepted on :18-July-2018
Published online on: 03-08-2018
Plagiarism Check: Yes
Reviewed by: Hesham N
Second Review by: Farzaneh Fesahat
Final Approval by: Dr. Annapaola Zito
Suhad A.Ibrahim1,2 , Jwan A. Zainulabdeen1 and Hameed M. Jasim2
, Jwan A. Zainulabdeen1 and Hameed M. Jasim2
1Department of Chemistry, College of science, University of Baghdad, Baghdad, Iraq.
2Department of Biotechnology, College of Biotechnology, Al-Nahrain University Baghdad, Iraq.
Corresponding Author E-mail: su_aziz2015@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1502
Abstract
This study aims to determine the relationship between spermidine (SPD) and spermine (SPM) with the incidence of atherosclerosis in population of Iraqi patients in comparison with controls. A total of 128 atherosclerosis patients (64 male and 64 female) were included in the present study (aged 45-70 years). They were categorized into two groups: a group that underwent percutaneous coronary intervention catheterization (PCI) and a group that underwent diagnostic catheterization (DIG). 64 age matched, apparently healthy individuals (showing no symptoms of heart disease) of both men and women were also included in the control group (C). Sera were used as samples of the present study; the main measurements included SPD and SPM levels using the HPLC method. Results showed that serum levels of SPD were 631.53 ± 35.637, and 540.49 ± 44.564µg/ml in PCI and DIG patients, respectively. These levels showed significant increases (p˂0.001) when compared to their levels (29.162 ± 6.151µg/ml) in sera of healthy controls. On the other hand, the SPM levels were (159.98 ± 15.981, and 169.85 ±14.606 µg/ml) in PCI and DIG patients, respectively, which also show significant increases (p˂0.001) when compared with the SPM levels (24.26 ± 4.613 µg/ml) in the control group. Moreover, the changes in SPD and SPM levels differed significantly (p˂0.001, and p˂0.05, respectively) according to gender in patient groups. Moreover, it was found that there were positive correlations between sera levels of SPD and SPM in atherosclerosis patients with the levels of white blood cells (WBC) (r=0.58, p˂0.05, r=0.55, p˂0.05), neutrophils (r=0.54, p˂0.05, r=0.51, p˂0.05), lymphocytes (r=0.46, p˂0.05, r=0.48, p˂0.05) and red blood cells (RBC) (r=0.43, p˂0.05, r=0.60, p˂0.01). These results demonstrated that SPD and SPM levels were altered in sera of atherosclerosis patients when compared with the control group. In addition, the data indicated that these patients’ gender played a role in their levels. Generally, correlations were observed among the SPD and SPM levels, the white blood cell differential, and the red blood cells in the sera of atherosclerosis patients.
Keywords
Atherosclerosis; Spermidine; Spermine;White Blood Cells Counts
Download this article as:| Copy the following to cite this article: Ibrahim S. A, Zainulabdeen J. A. Jasim H. M. The Significance of Spermidine and Spermine in Association with Atherosclerosis in Sera of Iraqi Patients. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Ibrahim S. A, Zainulabdeen J. A. Jasim H. M. The Significance of Spermidine and Spermine in Association with Atherosclerosis in Sera of Iraqi Patients. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=21815 |
Introduction
Coronary atherosclerosis occurs when the arteries are damaged. It constricts and reduces blood flow within the artery leading to the heart muscle especially when fatty substances accumulate on the inner wall of the coronary arteries.1 In some cases, atherosclerosis leads to the cessation of blood flow due to excessive accumulation of fatty and amino acids.2 It has been recognized as an active and inflammatory process. Atherosclerosis is considered the major cause of morbidity and mortality in industrialized countries.3 Endogenous and exogenous risk factors such as obesity, metabolic syndrome, and type 2 diabetes, result in an array of metabolic and vascular events which can cause the development of atherosclerosis.4,5,6 These factors can alter the content of polyamines (PAs) in the heart and thus become a potential cause of an increase in cardiovascular disease.7 PAs are ubiquitous polycations and are considered aliphatic compounds that contain nitrogen, and they are fully protonated under physiological conditions.8
Spermidine (SPD) and spermine (SPM) are the most common members of the PA family which have low molecular weight.9 In mammals, the main sources of PAs are cellular synthesis, food intake, and microbial synthesis in the gut.10 Also, PAs are induced in response to many physico-biochemical stresses such as osmolality and reactive oxygen species (ROS).11 The rate-limiting enzyme in PA biosynthesis is ornithine decarboxylase; putrescine is synthesized from ornithine which is formed from arginine (ARG) by arginase.12 As a consequence, SPD is synthesized from putrescine by spermidine synthase, then SPD is converted to SPM by spermine synthase.7 Polyamines are essential for all cellular life of eukaryotic and prokaryotic cells for the purpose of differentiation and cell growth.13 They also impact DNA replication, gene expression, protein synthesis, and stabilization of lipids.12 It was found that intake of PAs reduces the incidence of cardiovascular disease.12 But over-production or over-intake of these PAs is toxic to cells as it facilitates cell death by an oxidative mechanism.14 In some cases, it may cause some symptoms such as headaches, nausea, hypotension or hypertension, and cardiac palpitations.5 Thus, PAs homeostasis is essential, because their catabolic pathways of cellular PAs by polyamine oxidases, spermine oxidase or acetyl polyamine oxidase, increase cellular oxidative stress and generate a reactive toxic aldehyde, acrolein and hydrogen peroxide.15,16 Multiple abnormalities in the control of PA metabolism might be involved in different diseases such as stroke, other neurological diseases, liver disease, chronic renal failure,17 and diabetes in breast cancer patients.18 Meanwhile, other studies have shown that PAs have been involved in many diseases related to atherosclerosis, such as cardiac hypertrophy,19,20 cardiac tissues with heart failure,21,11 and myocardial ischemia/reperfusion injury.22 For a better understanding of the role of PAs in atherosclerosis, research should be conducted in specific contexts. Therefore, the purpose of this study is to measure the concentrations of SPD and SPM in the sera of Iraqi patients with atherosclerosis and their correlation with this disease and white blood cell count, in addition to the difference of gender on polyamine concentration.
Materials and Methods
Study Subjects
A total of 128 patients suffering from atherosclerosis symptoms were enrolled in this study. Patients who underwent (PCI catheterization), cardiac troponin I test (cTnI) were categorized as percutaneous coronary intervention catheterization; positive troponin I (PCI group, n=64). Patients who underwent diagnostic catheterization were categorized as negative Troponin I (DIG group, n=64). Patients were compared to apparently healthy control subjects (group C, n=64). Each group contains 32 males and 32 females with ages ranging between 45 to 70 years, and a body mass index ranging between 25 to 30 kg/m2. Exclusion criteria included: smokers, a history of coronary artery disease (CAD), inflammatory disease, infection, hematological disease, hepatic disorder, renal dysfunction, cancer, and autoimmune disease. These tests were performed at Al-Sheikh Zayed and Ibn Al-Nafees Hospital (Coronary Care Unit and Catheterization Unit) in Baghdad, Iraq. This study protocol was approved by the Ethics Committee of the College of Science/University of Baghdad.
Collection of Blood Samples
A volume of 7 ml of blood samples was collected from each case study and divided to two parts. The first part (2 ml) of blood samples were placed in EDTA tubes for hematological examination by using hematology Abbott analyzer/Ruby/USA. The remaining blood was placed in plain tubes and left to stand for 10 minutes at room temperature until clot formation. These tubes were then centrifuged at 3000 rpm for 10 minutes. Serum samples obtained after centrifugation were kept at -20°C for determination of SPD and SPM concentrations.
Polyamines Determination
Levels of SPD and SPM were determined using a SyKNM model, high-performance liquid chromatography (HPLC) with UV-visible detection at (λ=254 nm).23,24,25 The stationary phase was, Octa Dislocate (ODS), shim-pack C18, 5 µm particles in column (25*0.46). The mobile phase was methanol-water (60:40). Aliquots of 20 µl sample loop of benzoylated standard PAs and derivatized serum PAs were injected and run isocratically at a flow rate of 1ml/min.
Chromatographic Separation of Spermidine and Spermine Derivative
Throughout the current project, HPLC-UV has been used for the estimation of both PAs which gave significant peaks, as shown in Figure 1A & B.24 The retention time of SPD and SPM under the used experimental conditions was 10.50 and 6.12 minutes, respectively.
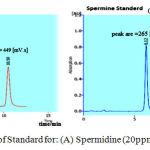 |
Figure 1: Chromatogram of Standard for: (A) Spermidine (20ppm), (B) Spermine (20ppm).
|
Preparation of Spermidine and Spermine Standard Curves
Serial dilution of both SPD and SPM standards were prepared. Their final concentrations ranged between (2.5-50 µg/ml). 10µl of each concentration was injected in Autosampler HPLC. The area under the peak (AUC) was recorded for all samples, as shown in Figure (2A&B). The following equations (Y==24.37X+1.5699) and (Y==12.6X+26.241) were used to calculate the concentration of SPD and SPM, respectively for each studied sample.
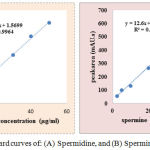 |
Figure 2: Standard curves of: (A) Spermidine, and (B) Spermine.
|
Derivatization of Serum Polyamines
Serum SPD and SPM were derivatized by adding 1ml of benzoyl chloride (2% in absolute methanol) to 0.5 ml of serum sample in screw-capped test tubes. After a milky- color solution was formed, a 1 ml of 2 M sodium hydroxide was added and vortexed for 30 seconds and incubated at 37ºC for 15 minutes. To terminate the reaction, a 2 ml of saturated sodium chloride solution and 3 ml of diethyl ether were added, vortexed for 1 minute, and centrifuged at 3000 rpm for 10 minutes. In order to perform separation, the organic layer containing benzoylated PA was transferred to another screw-capped tube and left to evaporate for dryness. The residual was dissolved in 2 ml of methanol solution and filtered to remove particulates. The filtrate was used for determining the SPD and SPM concentration by using Auto sampler-HPLC.
Statistical analysis
A standard curve was used to determine the concentration of spermidine and spermine in patient and control groups by using Excel 2013. One-way ANOVA was used to compare the differences between SPD and SPM among groups. Statistical comparison was performed by paired or unpaired Student t-test to compare between male and female patients. The significance level was set up at p˂0.05. All data are presented as a mean ± standard error (S.E.M).
Results
In the current work, blood samples were used to estimate the changes in total WBC counts with its differential as well as RBC counts in categorized patients (group PCI, group DIG), and in group C as illustrated in table (1). The result shows that a highly significant increase of p< 0.01 was observed in mean ± SE of total WBC counts and lymphocytes in both patient groups when compared with group C (7.53b ± 0.41, 2.31 b ± 0.19, respectively), group PCI (8.87 a ± 0.36, 3.01 a ± 0.12, respectively), and group DIG (8.64a ± 0.51, 3.15a ± 0.31, respectively). The mean ± SE of basophils in patients DIG (0.086ab ± 0.02) was significantly more than patients in group PCI by p < 0.01 (0.102a ± 0.008) and group C (0.070b ± 0.006). The differences in neutrophils, monocytes, and RBC levels were non–significant (p < 0.05) among groups in this study.
Table 1: Total White blood cells (WBC) Differential and Red blood cells (RBC) in the blood of the three studied groups.
| Group | Mean ± SE | ||||||
| WBC (×109/L) | Neutrophil (%) | Lymphocyte (%) | Monocyte (%) | Eosinophil (%) | Basophile (%) | RBC (×106/µl) | |
| PCI | 8.87a ± 0.36 | 4.86a ± 0.26 | 3.01a ± 0.12 | 0.576a ± 0.04 | 0.314a ± 0.07 | 0.102a ± 0.008 | 4.89a
± 0.12 |
| DIG | 8.64a ± 0.51 | 4.70a ± 0.29 | 3.15a ± 0.31 | 0.501a ± 0.05 | 0.197a ± 0.03 | 0.086ab ± 0.02 | 4.69a
± 0.08 |
| Control | 7.53b ± 0.41 | 4.55a ± 0.34 | 2.31b ± 0.19 | 0.521a ± 0.03 | 0.250a ± 0.07 | 0.070b ± 0.006 | 4.72a
± 0.10 |
| LSD value | 1.244 * | 0.868 | 0.613 * | 0.122 | 0.181 | 0.0242 * | 0.304
NS |
| P-value | 0.0502 * | 0.769 | 0.0155* | 0.452 | 0.456 | 0.027 * | 0.392 |
*: Significant using ANOVA test at 0.05 level of significance, **: < 0.01 level of high significance, NS: Non- Significant.
When SPD and SPM concentrations were measured in the sera of patients under diagnostic (DIG), percutaneous intervention catheterization (PCI) patients, and control groups, the results in (Table 2) show that there were highly significant increases of p < 0.01 in SPD concentrations for PCI and DIG groups (mean ± SE, 631.53a ± 35.637, and 540.49a ± 44.564, respectively, when compared with the levels in group C, 29.162b ± 6.151). The mean ± SE of SPM in both patient groups was significantly more by p < 0.01 (169.85a ± 14.606 and 159.98a ± 15.981 for the DIG and PCI patients, respectively) when compared with group C (24.26b ± 4.613). Non-significant differences (p >0.05) were found between DIG and PCI groups.
Table 2: Mean ± stander error of spermidine and spermine levels in sera of the three studied groups.
| Group | Mean ± Standard Error | |
| Spermidine (µg/ml) | Spermine (µg/ml) | |
| PCI | ↑ 631.53a ± 35.637 | 159.98a ± 15.981 |
| DIG | ↑ 540.49a ± 44.564 | 169.85a ± 14.606 |
| C | 29.162b ± 6.151 | 24.26b ± 4.613 |
| LSD value | 165.09 | 51.979 |
| P-value | 0.0001** | 0.0001 ** |
Different letters mean significant differences (p< 0.01) and the same latters mean there were non-significant differences (p>0.05) between the three studied groups.
To follow up on the role of gender in SPD and SPM concentrations, the three studied groups were subdivided by men and women. Table 3 illustrates that the mean ± SE of SPD and SPM concentrations were increased significantly by p˂ 0.001 in the sera of females with PCI (521.02 ± 93.16 and 133.32 ± 29.20, respectively) when compared with polyamine concentration in males (411.294 ± 168.64, 105.44 ± 40.83, respectively). In contrast, the concentrations were significantly higher by p˂ 0.001 in the sera of male individuals with DIG (468.75 ± 10.41 and 139.18 ± 32.28, respectively) when compared with female patients (377.87 ± 126.66 and 110.93 ± 47.16, respectively). The concentrations of SPD and SPM in the sera of group C women (47.08 ± 16.29), (45.43 ± 29.81) were non-significantly higher than seen in group C men (11.99 ± 6.32), (11.74 ± 6.31), respectively.
Table 3: Mean ± stander error of spermidine and spermine concentration according to the gender in the studied groups.
| Parameters | Spermidine (µg/ml)
Mean ± Standard Error |
Spermine µg/ml
Mean ± Standard Error |
P-value | ||
| Male | Female | Male | Female | ||
| PCI | 411.29 ± 168.64 | 521.02 ± 93.16 | 105.44 ± 40.83 | 133.32 ± 29.20 | ≤ 0.01 ** |
| DIG | 468.75 ± 10.41 | 377.87 ± 126.66 | 139.18 ± 32.28 | 110.93 ± 47.16 | ≤ 0.05 * |
| C | 11.99 ± 6.32 | 47.08 ± 16.29 | 11.74 ± 6.31 | 45.43 ± 29.81 | ≥ 0.05 |
S: Significant *(p ≤ 0.05) and ** (p ≤ 0.01), NS :Non-significant (p≥ 0.05)
Correlations among hematological factors with both SPD and SPM were evaluated as presented in Table (4). Serum SPD and SPM were significantly correlated with total WBC counts, neutrophils, lymphocytes, eosinophils, and RBCs, while correlations among monocyte and basophil levels in patients with atherosclerosis were non-significant in regard to both SPD and SPM.
Table 4: Correlation coefficient between spermidine and spermine and other parameters in atherosclerosis patients.
| Parameters | Correlation coefficient-r | |
| Spermidine | Spermine | |
| WBC | 0.58 * | 0.55 * |
| Neutrophil (%) | 0.54 * | 0.51 * |
| Lymphocyte (%) | 0.46 * | 0.48 * |
| Monocyte (%) | 0.07 NS | -0.003 NS |
| Eosinophil (%) | 0.64 ** | 0.44 * |
| Basophile (%) | 0.25 NS | 0.12 NS |
| RBC | 0.43 * | 0.60 ** |
* (P<0.05), ** (P<0.01), NS: Non-Significant.
Discussion
In this study, comparisons among three groups in the levels of some hematologic parameters, as well as PAs (SPM and SPD), were conducted. The groups were categorized as follows: percutaneous coronary intervention catheterization patients (PCI), diagnostic catheterization patients (DIG), and apparently healthy controls (C). The results indicated a significant increase in total WBCs, lymphocytes, and basophils in patient groups when compared to controls. Such increases observed in atherosclerosis patients were explained by Ates AH, et al., 2011 and Mozaffarian, 2006, as they argued that an increase in total WBCs plays an important role in inflammation and is associated with the presence, severity, and extent of coronary atherosclerosis.26,27 In addition, Nettleton et al., 2006 pointed out the importance of inflammation in the mechanism of atherosclerosis and CVD.28
In the current work, SPM and SPD concentrations were measured according to benzoyl chloride derivation method using HPLC-UV, which is considered a stable and permitted detection method of moderate concentration of PAs.23,24 According to present findings, SPD and SPM concentrations were statistically higher in the sera of both PCI and DIG patient groups when compared to group C, as shown in Table 1. That means the synthesis pathway of PAs (SPD and SPM) is active. Increasing and decreasing concentrations of PAs play a role in the pathogenesis of many diseases in cardiac tissue or cells after ascending aortic stenosis, stress, and physical exercise.11 Therefore, it is important to maintain an equilibrium level of PAs, because a high production of PAs can activate the oxidative catabolic pathways. However, their catabolic pathways generate reactive oxidants by a number of oxidases. SPD is more potent than SPM as well as its metabolites, particularly those from SPM. They can cause significant toxicity with damage to proteins, DNA, and other cellular components.15
Such an increase in PA synthesis means disturbances in PA homeostasis and may lead to many diseases, such as cardiovascular diseases, as confirmed by other studies; this suggests that PAs can be introduced from cells and cause biological changes in cardiomyocytes.29,30
On the other hand, the consumption of PAs’ precursor amino acid ARG (as confirmed by the observed increase in PAs levels in patients groups when compared with group C) cause changes in ARG metabolism, which is an important factor in a variety of diseases.12 Although the contribution of ARG expression to atherosclerosis in each of the vascular cell types is still unknown, quantitative variations in ARG expression and/or activity seem to modulate the overall susceptibility to the disease.12 The other explanation for such a result may be due to ornithine, the primary metabolite of the ARG pathway; a previous study suggests that ornithine may play an important part in atherogenesis.11 In addition, various metabolites of ornithine play an important part in the matrix and cellular composition of the atherosclerosis plaque, a critical feature for plaque vulnerability.30 Several studies have shown that PAs in adipose tissue cells affect lipid and glucose metabolism, as in the case of insulin. It was found that insulin-stimulated tyrosine kinase (IRTK) activity stimulated by insulin can be activated by Mg+2, SPD, and SPM in the absence of insulin.4,6,31
Despite having more comorbid risk factors than men, women have less extensive coronary artery diseases. In previous studies, the result of the role of the individual’s gender in atherosclerosis focused on a composition of atherosclerotic plaque differences in men and women with acute coronary syndromes (ACS),32,33 which, as this study suggests, may be the reason for the higher levels of SPD and SPM in male DIG patients when compared with women in the DIG group. In contrast, the higher levels of SPD and SPM in female PCI patients when compared with the men in the DIG group may be related to the reduction of ovarian function in menopausal women.34
This study is the first trial to evaluate the SPD and SPM levels in this disease (atherosclerosis) for Iraqi patients who underwent diagnostic or percutaneous catheterization. According to this study, there is a clear finding that PA levels increase in the sera of atherosclerosis patients and also in individuals who have the symptoms of this disease. The concentrations of PAs are also affected by the individual’s gender. Additional work in this regard may provide important benefits in the early diagnosis and treatment of atherosclerosis disease.
Acknowledgement
We would like to acknowledge all participated patients and healthy individuals, who were very helpful in giving information and blood samples.
References
- Heart I. Foundation, Step by Step through Cardiac Catheterization and Angioplasty, AstraZeneca (Pharmaceuticals) Ireland Ltd. 2011;432-787.
- Ignarro L.J, Balestrieri M.L, Napoli C. Nutrition, physical activity, and cardiovascular disease: an up-date. Cardiovascular Research. 2007;73(2):326-340.
CrossRef - Lichtenstein A.H, Appel L.J,Brands M,Carnethon M, Daniels S, Franch H. A, Franklin B, Kris-Etherton P,Harris W.S, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Horn V.L,Winston M,Wylie- Rosett J. Summary of American heart association diet and life-style recommendations revision. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(10):2186-2191.
CrossRef - Tessari P, Cecchet D,Cosma A, Puricelli L, Millioni R, Vedovato V, Tiengo A. Insulin resistance of amino acid and protein metabolism in type 2 diabetes. Clinical Nutrition. 2011;30(1):267-272.
CrossRef - Shah P.K. Screening asymptomatic subjects for subclinical atherosclerosis: Can we, does it matter, and should we? J Am Coll Cardiol. 2010;56:98-105.
CrossRef - Cubbon R.M, Kahn M.B, Wheatcroft S.B. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci. 2009;117:173-190.
CrossRef - Hamana K, Matsuzaki S. Polyamines as a chemotaxonomic marker in bacterial systematics. Rev. Microbiol. 1992;18:261–283.
- Teti D, Visalli M and McNair H. Analysis of polyamines as markers of (patho) physiological conditions. J. B. 2002;781:107-149.
- Erik H, Tsikas D and Brunner G. Spermidine for a long, dementia-free life? Global Journal of Pharmacy & pharmaceutical Science. 2017;2(1):1-8.
- Reynolds K, Chin A, Lees K. A. Meta-analysis of the effect of soy protein supplementation on serum lipid. Am J Cardiol. 2006;98(5):633-640.
CrossRef - Giordano E, Flamigni F, Guarnieri C. Polyamines in cardiac physiology and disease. Open Heart Failure Journal. 2010;3:25-30.
CrossRef - Moinard C, Breuillard C and Charrueau C. L-Arginine metabolism ımpairment in sepsis and diseases: causes and consequences, in L-Arginine in Clinical Nutrition, Berlin, Germany: Springer International Publishing. 2017;145-158.
CrossRef - Stephenson A.H, Christian J.F, Seidel E. R. Polyamines regulate eukaryotic initiation factor 4E binding protein 1 gene transcription. Biochemical and Biophysical Research Communications. 2004;32(3):204-212.
CrossRef - Gnarro M, Balestrieri L, Napoli C. Nutrition, Physical Activity, and Cardiovascular Disease: an up-date. Cardiovascular Research. 2007;73(2):326-340.
CrossRef - Hoet P.H, Nemery M.B. Polyamines in the lung: Polyamine uptake and polyamine-linked pathological or toxicological conditions. Lung Cell.Mol.physio. 2000;278:417-433.
- Milton S. Hershey Medical Center, Pennsylvania State University College of Medicine, Hershey, Toxicity of Polyamines and Their Metabolic Products. Pennsylvania 17033, United States, Department of Cellular and Molecular Physiology, Res. Toxicol. 2013;26(12):1782–1800.
- Saito A, Takagi T, Chung T.G, Ohta K. Serum levels of polyamines in patients with chronic renal failure. Kidney Int SuppI. 1983;16(S):234-7.
- Kenan V.C, Kapancik S, Kacan T, Baloglu S.K,Kapancikand S, Kilcgun H Serum levels of polyamine synthesis enzymes increase in diabetic patients with breast cancer. Turkey. 2017.
- Hee M.P and Igarashi K. Polyamines and Their Metabolites as Diagnostic Markers of Human Diseases Biomol Ther. 2013;21(1):1-9.
- Celik V.K, Ersan E.E,Kilicgun H, Kapancik S, Ersan S. Agmatine mediated hypertonic stress development in Schizophrenia: A Novel study. Neuropsychiatry. 2016;6:184-189.
CrossRef - Meana C; Manuel J.R, Bordello C,Suarez L, Bordallo J,Sanchez M. Correlation between endogenous polyamines in human cardiac tissues and clinical parameters in patients with heart failure. J cell Mol Med Spain. 2016;20( 2):302-312.
- Ya-Jun Z,Chang-Qing X,Wei-Hua Z, Zhang L,Shu-Ling B,Huang Q, Hong-Li S, Quan-Feng L, Yan-qiao Z,Tian Y; Wang R; Bao-Feng Y, Wei-Min L. Role of polyamines in myocardial ischemia /reperfusion injury and their interactions with nitric oxide. European journal of pharmacology, Elsevier. 2007;562:236-246.
CrossRef - Rajat S; Raghuveer S.C,Bashir S.M, Castro E. An improved high-performance liquid chromatographic method for identification and quantization of polyamines as benzoylated derivatives. American Journal of Analytical Chemistry. 2011;2:456-469.
CrossRef - Schenkel E, Berlaimont V, Dubois J. Improved high performance liquid chromatographic method for the determination of polyamines as their benzoylated derivatives: application to P388 cancer cells. J. Chromatogr B. 1995;668:189-197.
CrossRef - Sethi R, Raghuveer S.C,Bashir S, Mauro E. Castro, an Improved High Performance Liquid Chromatographic Method for Identification and Quantization of Polyamines as Benzoylated Derivatives. American Journal of Analytical Chemistry. 2011;2:456-469.
CrossRef - Ates A.H, Canpolat U, Yorgun H. Tital white blood cell count is associated with the presence, severity and extent of coronary atherosclerosis detected by dual-source multislice computed tomographic coronary angiography. Cardiol J. 2011;18:371-377.
- Mozaffarian D. Trans fatty acids-effects on systemic inflammation and endothelial function. Atheroscler Suppl. 2006;7(2):29-32.
CrossRef - Nettleton J.A,Steffen L.M ,Mayer-Davis E.J, Jenny N.S, Jiang R, Herrington D.M, Jacobs D.M, Jacobs D.R. Jr, Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). J clin Nutr. 2006;83(6):1369-1379.
CrossRef - Felicia M.F, Acquafredda A,Tesse R ,Luce V, Ventura A,Maggialetti N, Monteduro M, Giordano P,Cavallo L. Polyamines: therapeutic perspectives in oxidative stress and inflammatory diseases. September. 2017;49(9):1457-1468.
- Robert A. CASERO Jr and Anthony E. PEGG;” Polyamine catabolism and disease”. Biochem J. 2010;421(3):323–338.
CrossRef - Ralph P, Lingxiang Ye. Regulation of heart insulin receptor tyrosine kinase activity by magnesium and spermine. Molecular and Cellular Biochemistry. 2005;277:7-17.
CrossRef - Alexandra J. Gender and the extent of coronary atherosclerosis.
- Mosca L, Benjamin E.J, Berra K, Bezanson J.L,Dolor R.J, Lloyd-Jones D.M. Effectiveness based guidelines for the prevention of cardiovascular disease in women, update: a guideline from the American Heart Association. Circulation. 2011;123:1243-1262.
CrossRef - Howard B.V, Horn V.L, Hsia J. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification. Trial. JAMA. 2006;295(6):655-666.
CrossRef







