Manuscript accepted on :30-Aug-2018
Published online on: 06-09-2018
Plagiarism Check: Yes
Reviewed by: Dini Damayanti
Second Review by: ABEL ABBAS
Final Approval by: Prof. Juei-Tang Cheng
Supri I. Handayani1, Rahmiati1, Lisnawati Rahmadi1, Rosmalena2 and Vivitri D. Prasasty3
1Department of Anatomical Pathology, Faculty of Medicine, University of Indonesia, Jalan Salemba Raya 6, Jakarta 10430, Indonesia.
2Department of Medical Chemistry, Faculty of Medicine, University of Indonesia, Jalan Salemba Raya 6, Jakarta 10430, Indonesia.
3Faculty of Biotechnology, Atma Jaya Catholic University of Indonesia Jalan Jenderal Sudirman 51, Jakarta 12930, Indonesia.
Corresponding Author E-mail: vivitri.dewi@atmajaya.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1492
Abstract
Hypoxia inducible factor 1 alpha (HIF-1α) regulates cell growth and differentiation which is implicated in human cancers. HIF-1α activates its cascade carcinogenesis mechanism in cancer cells. It is well-understood that signaling is initiated by HIF-1α receptor. Overexpression of HIF-1α is associated with several different human cancers, including breast cancer, lung cancer and colon cancer. Thus, HIF-1α becomes potential target of therapeutic approach in developing HIF-1α inhibitors. The aim of this research is to investigate potential inhibitors which are known as Acetogenins (AGEs) isolated from Annona muricata against HIF-1α. In order to achieve this goal, chemical structures of all compounds were retrieved from PubChem database. Molecular docking was performed by AutoDock Vina program and the resulting binding modes were analyzed with AutoDock Tools program. Among all the compounds, murihexocin A showed the best binding modes compared to other two inhibitors based on the lowest binding energies (LBE = -7.9 kcal/mol) as high as gefitinib. This was indicating that murihexocin A has favorable interaction with the essential amino acid residues at catalytic site of HIF-1α. Drug-likeness calculation of AGEs were also performed. These in silico results could be beneficial as a compound model for further studies in-vitro and in-vivo.
Keywords
Acetogenins; Annona Muricata; Drug-Likeness; HIF-1α; Inhibitor, Molecular Docking
Download this article as:| Copy the following to cite this article: Handayani S. I, Rahmiati R, Rahmadi L, Rosmalena R, Prasasty V. D. Molecular Docking and Drug-Likeness for the Identification of Inhibitory Action of Acetogenins from Annona Muricata as Potential Anticancer Against Hypoxia Inducible Factor 1 Alpha. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Handayani S. I, Rahmiati R, Rahmadi L, Rosmalena R, Prasasty V. D. Molecular Docking and Drug-Likeness for the Identification of Inhibitory Action of Acetogenins from Annona Muricata as Potential Anticancer Against Hypoxia Inducible Factor 1 Alpha. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22320 |
Introduction
Hypoxia inducible factor-1 (HIF-1) is a transcription factor that controls the expression of gene that involved in tumorigenesis and metastases of malignancies.1,2 The HIF-1α level could be enhanced within breast oncogénesis, and it closely related with other tumor biomarkers.3 HIF-1α plays an important role in binding the consensus sequence 5′-RCGTG-3′ (which R is purine) at the response elements of hipoxia to target genes.4 The transcription process of various genes are activated by HIF-1, including glycolytic enzymes, gluconeogenesis, mediating glucose transporters, growth factors, high-energy phosphate metabolism, heme metabolism, iron transport, erythropoiesis, synthesis of nitric oxide, and regulation of vasomotor. Therefore, HIF-1 possibly promotes the tumor cell viability in hypoxic circumstances.5,6.
Hypoxia induces tumor cell proliferation, metastasis, and the rate of cell apoptosis.7 Moreover, HIF-1 is considered as a starting point of angiogenic process in tumor cells by transcription activation of cancer-related gene, such as vascular endothelial growth factor (VEGF) gene.8 The level of HIF-1α escalates the pathological stage which is higher in poorly differentiated lesions than in well-differentiated lesions.3 The enhanced levels of HIF-1α are strongly related with high proliferation and enhanced ER as well as VEGF expressions.9 Therefore, the high level of HIF-1α has potency in associating with further massive tumors.3
Natural bioactive compounds which are derived from plants have been used for maintaining health and remedies in many years. The phytochemical constituents in plants have been a critical pipeline for the discovery of bioactive substances in pharmaceutical field.10 A. muricata or Graviola has been greatly pressumed to have valuable natural products that play important role in affecting anticancer activity.11 A. muricata leaves have been used to investigate of numerous numbers of human diseases, including cancers.10 The highly constituents screening are most possibly affected by its major bioactive components known as annonaceous Acetogenins (AGEs). 12 Many studies reported that isolated AGEs from different extracts of the plants have significant antiproliferative effects against various cancer cell lines.10 However, some of these studies have defined the staple mechanism of action. Recent in-vitro studies showed inhibition action of ethyl acetate extract from A. muricata leaves combating lung cancer cells (A549) and colon cancer cells (HCT-116 and HT-29).10,13 The leaf extract was capable of inducing colon carcinoma and lung cancer cells apoptosis by way of mitocondrial route. This antiproliferative effect was associated with cell cycle involved in the G1 phase. Moreover, the migration and invasion of colon cancer cells were significantly halted by the leaf extract.14-16
The aim of this research is to determine the inhibition mechanism by bioactive compounds of A. muricata interact with HIF-1α. To study the binding interactions of bioactive compounds with HIF-1α through molecular docking methods. Computational methodologies have become a crucial component in drug discovery program, which involves identification to lead optimization. Molecular modeling is one of the methodologies primarily used as hit identification tool when only structure of target and its active or binding site are available.17 Docking method is an energy-based scoring function which identifies the energetically most favorable ligand conformation that binds to the target.18
Method
Protein Structure Preparation
The amino acid sequence of HIF-1α (Entry PDB code : 4z1v) was retrieved from RSCB Protein Database.19 The attached ligand in the protein structure was removed from the binding site and saved to a new file format: pdbqt. The Gasteiger charges and the solvation condition were added to the protein structure using the AutoDockTool.20
Ligand Structures Preparation
Ligands which are AGEs consisting of eight 3D structures of natural bioactive compounds originally belong to A. muricata and one anticancer drug for molecular docking experiments and their conformational energy were minimized by using MMFF94 force field. 8 molecules of AGEs. The molecule structures are retrieved from PubChem database (Fig. 3). The structures were scored based on their physicochemical properties under Chemicalize (ChemAxon) and Molsoft platforms.21,22 These physicochemical properties are important for developing drug candidate in every stages from design to pre-clinical study.
Drug likeness analysis of A. muricata bioactive compounds
3D structures of Cinchona alkaloids were analyzed using a program based on the physicochemical properties, Molsoft Drug – Likeness. Determination of physicochemical properties is important in the development of drug candidates in all stages ranging from study design through pre- clinical trials.22
Molecular Docking of HIF-1α and A. Muricata Bioactive Compounds
The three dimension structure of protein and ligands were prepared in pdb format. Molecular docking simulation was run by Autodock Vina (Vina, The Scripps Institute).23 The AutodockTools (ADL) was utilized in minimizing energy and adding the partial charges of polar hydrogens of receptor (protein). The ligands were prepared with flexible torsion angles and the protein was prepared in a static (rigid) form. Furthermore, protein and ligands were kept in pdbqt formats which suitable for docking simulation. The affinity binding were calculated as total intermolecular energies (kcal/mol) which involved hydrogen bond, Van Der Walls force, desolvation and electrostatic energies. On the other hand, the apropriate torsion angles of ligand is also induced as internal ligand energy. The docking program evaluated the lowest binding energy (LBE) to obtain the best binding mode. The Root-Mean-Square Deviation (RMSD) which less than 2.0 Å was scored during running docking program.
Results
Bioactive compounds isolated from A. muricata , including annomuricin A, annomuricin B, annomuricin C, annomuricin E, annomutacin, murihexocin A, murihexocin B, murihexocin C, and gefitinib (Figure 1) were docked into binding pocket of HIF-1α.
 |
Figure 1: AGEs compound of A. muricata leaves: a. annomuricin A; b. annomuricin B; c. annomuricin C; d. annomuricin E; e. annomutacin; f. murihexocin A; g. murihexocin B; h. murihexocin C; i. gefitinib.
|
The lowest binding energy (LBE) to the target protein was murihexocin A (-7.9 kcal/mol). The binding interactions between A. muricata bioactive compounds with of HIF-1α binding pocket residues were analysed as shown in Table 1.
Table 1: Binding interactions between A. muricata bioactive compounds with of HIF-1α binding pocket residues.
| Compound | LBE (kcal/mol) | H-bonding | Hydrophobic Interaction with HIF-1α residues |
| Annomuricin A | -6.7 | 3 | Tyr-93, Tyr-102, Asp-104, Leu-186, Leu-188, Gln-147, His-199, Phe-207, Ile-281, Asp-201, Arg-238, Gln-239, Trp-296, His-279 |
| Annomuricin B | -7.2 | 2 | Thr-149, Thr-183, Ser-184, Leu-186, Trp-296, Ile-281, Gln-203, Asp-201, Arg-238, His-199, Gln-239, Tyr-102, Thr-196, His-279 |
| Annomuricin C | -7.1 | 2 | Ser-184, Asn-294, Asn-205, Trp-296, Tyr-93, Tyr-102, Arg-238, Asp-104, Asp-237, Gln-239, Tyr-103 |
| Annomuricin E
|
-7.3 | 2 | Trp-296, Gln-203, Asp-201, Arg-238, Asp-237, Pro-235, Gln-239, Tyr-102, Ile-281, Leu-188, Asn-294, Thr-196, Tyr-103, Thr-196, Lys-214 |
| Annomutacin | -6.9 | 0 | Thr-183, Ser-184, Gln-203, Trp-296, Leu-186, Arg-238, His-199, Tyr-102, His-279, Thr-196, Ile-281, Gln-147, Trp-296, Ser-184 |
| Murihexocin A | -7.9 | 1 | Thr-183, Trp-296, Tyr-102, His-199, Gln-147, Gln-203, Glu-202, Ser-184, Pro-235, Gln-239, Tyr-103, Ile-281 |
| Murihexocin B | -6.7 | 0 | Tyr-93, Asp104, Tyr-102, Trp-296, Leu-166, Ser-184, Arg-238, Asp-201, Gln-203, Glu-202, Tyr-93, Asp-104, Gln-239 |
| Murihexocin C | -7.6 | 2 | Gln-147, Thr-196, His-199, Gln-203, Arg-238, His-279, Asn-294, Ser-184,
Phe-207, Gln-203, Trp-296, Leu-188 |
| Gefitinib
|
-7.9 | 2 | Gln-147, Thr-196, His-279, Asn-294, Ser-164, Gln-203, Arg-238, Gln-239, Tyr-102, Tyr-103, Phe-207 |
From docking result, murihexocin A compound interacted hydrophobically with Thr-183, Trp-296, Tyr-102, His-199, Gln-147, Gln-203, Glu-202, Ser-184, Pro-235, Gln-239, Tyr-103, and Ile-281 in the binding pocket of HIF-1α.
The molecular interaction of murihexocin A with HIF-1α is illustrated in Fig. 2. Murihexocin A interacted with HIF-1α was stabilized by hydrogen bond between nitrogen atom of carboxamide group of Ser-184 side chain with hydrogen from hydroxyl group of murihexocin A.
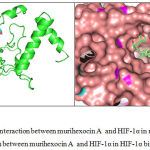 |
Figure 2: a. Complex interaction between murihexocin A and HIF-1α in ribbon-stick form; b. Complex interaction between murihexocin A and HIF-1α in HIF-1α binding pocket.
|
Drug likeness properties of A. muricata bioactive molecules and comercial anticancer drug molecule, gefitinib were calculated using Molsoft Drug – Likeness program.
Table 2: Drug likeness properties of A. muricata bioactive molecules and commercial anticancer drug molecule.
| Compound | Drug Likeness | Log P | Molecular weight (g/mol) | TPSA (A2) | Stereocenter number** | Violation of Lipinski`s Rule** |
| Annomuricin A | -0,08 | 5.65 | 646.40 | 28.29 | 0 | 1 |
| Annomuricin B | 0.04 | 5.7 | 628.88 | 65.52 | 0 | 1 |
| Annomuricin C | -0,99 | 4.81 | 648.42 | 112.24 | 0 | 1 |
| Annomuricin E | -0.24 | 4,33 | 646.47 | 28.29 | 0 | 0 |
| Annomutacin | 0.04 | 4.81 | 647.45 | 65.52 | 0 | 0 |
| Murihexocin A | -0,99 | 4,33 | 628.88 | 112.24 | 0 | 0 |
| Murihexocin B | -0.24 | 4.81 | 648.41 | 28.29 | 0 | 0 |
| Murihexocin C | 0.04 | 4,33 | 648.40 | 65.52 | 0 | 0 |
| Gefitinib | -0.24 | 4,33 | 446.15 | 56.07 | 0 | 0 |
Discussions
The analysis of molecular docking has shown that the selected bioactive compounds interacted at similar site as triterpene with a different binding mode. The calculated lowest binding energy (LBE) values of the protein-ligand complexes are exhibited in Table 1. LBE is combined energy of the intermolecular energy and the free energy torsion which indicating the likeable interactions and strong binding with main amino acid residues at the binding pocket of the receptor. On the other hand, LBE also performed the intermolecular energy which was calculated based on the set of total energy which involved hydrophobic interaction, hydrogen bond interaction, electrostatic potential and desolvation free energy.
In order to find the best lead as anticancer agent from A. muricata bioactive compounds, we evaluated drug likeness properties of eight AGEs compounds compared with one commercial anticancer drug compound, gefitinib. It was found that all bioactive compounds had one violation of Lipinski`s rule of five, based on molecular weight. All molecular weight was above 500 g/mol, which means too big as a drug. However, according to docking result, murihexocin A was the best lead as anticancer agent and it could be used as a model for further analysis both in-vitro and in-vivo.
Approximately 133 acetogenins (AGEs) from different medicinal plants, such as Annona muricata, Annona squamosa Linn., Asiminatriloba (paw paw), and Cherimolia were reported have in-vitro anticancer activities against various cancer cell lines. Some AGEs such as asimin, asiminecin, asiminocin, and asiminacin have shown exceptionally high cytotoxicity for malignancies in three major tissues: breast, lung, and colon. Moreover, in-vivo data have been documented along with the tumor cell types, animal used, route of administration and dosage information. Some AGEs including annonacin, desacetyluvaricin, bullatacin, and bullatalicin have demonstrated significant in-vivo tumor growth inhibitory activities.24-26
Conclusion
Medicinal plants play important roles in the development of modern therapeutic agents. This study conclusively demonstrated that A. muricata was a good natural source of various phytochemical constituents. On the basis of our results, it can be concluded that the annonaceous AGEs were powerful phytochemicals found in A. muricata, which offers protective effect against cancer. In agreement with the lowest binding energy, annomuricin A, annomuricin B, annomuricin C, annomuricin E, annomutacin, murihexocin A, murihexocin B, murihexocin C and gefitinib were found -6.1, -7.2, -7.1, -7.3, -6.9, -7.9, -6.7, -7.6 and -7.9 kcal/mol, respectively. Murihexocin A showed similar LBE value with gefitinib. Thus, murihexocin A was selected to be the best lead as anticancer agent in silico. These in silico results could be beneficial as a compound model for further experimentally in-vitro and in-vivo assays to elucidate the exact mechanism of inhibitory activity and to examine its potential therapeutic effects.
Conflict of Interest
There is no conflict of interest.
Acknowledgement
Authors would like to thank The Scientific Committee of Department of Anatomical Pathology, Faculty of Medicine, University of Indonesia for the support to accomplish this work.
References
- Harris A. L. Hypoxia—a key regulatory factor in tumour growth. Nature Reviews Cancer. 2002;2(1):38-47.
CrossRef - Semenza G. L. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3(10):721-732.
CrossRef - Bos R., et al. Levels of hypoxia-inducible factor-1α during breast carcino genes is. Journal of the national cancer institute. 2001;93(4):309-314.
CrossRef - Semenza G. L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625-634.
CrossRef - Seeber L. M., et al. The role of hypoxia inducible factor-1alpha in gynecological cancer. Critical reviews in oncology.hematology. 2011;78: 173-184.
CrossRef - Seeber L. M., et al. Necrosis related HIF-1α expression predicts prognosis in patients with endometrioid endometrial carcinoma. BMC cancer. 2010;10(1):307-314.
CrossRef - Muz B., de la Puente P., Azab F & Azab A. K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83-92.
CrossRef - Aldebasi Y. H., Rahmani A. H., Khan A. A & Aly S. M. The effect of vascular endothelial growth factor in the progression of bladder cancer and diabetic retinopathy. International journal of clinical and experimental medicine. 2013;6(4):239-251.
- Li Q., Ma R & Zhang M. Co Cl 2 increases the expression of hypoxic markers HIF‑1α, VEGF and CXCR4 in breast cancer MCF‑7 cells. Oncology letters. 2018;15(1):1119-1124.
- Moghadamtousi S. Z., et al. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. International journal of molecular sciences. 2015;16(7):15625-15658.
CrossRef - Rosdi M. N. M., Daud N. N. N. N. M., Zulkifli R. M & Ya’akob H. Cytotoxic effect of Annona muricata Linn leaves extract on Capan-1 cells. Journal of Applied Pharmaceutical Science. 2015;5(5):45-48.
CrossRef - Rupprecht J. K., Hui Y. H & McLaughlin J. L. An nonaceous ace to genins a review. Journal of natural products. 1990;53(2):237-278.
CrossRef - Yang C., et al. Synergistic interactions among flavonoids and ace togenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis. 2015;36(6):656-665.
CrossRef - Moghadamtousi S. Z., et al. A nnona muricata leaves induce G1 cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells. Journal of ethno pharmacology. 2014;156:277-289.
CrossRef - Pieme C. A., et al. An ti proliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC complementary and alternative medicine. 2014;14:516.
CrossRef - Najmuddin S. U. F. S., Romli M. F., Hamid M., Alitheen N. B & Rahman N. M. A. N. A. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC complementary and alternative medicine. 2016;16:311.
CrossRef - Arnold K., Bordoli L., Kopp J & Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195-201.
CrossRef - Kitchen D.B., Decornez H., Furr J. R & Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nature reviews Drug discovery. 2004;3(11):935-949.
CrossRef - Berman H., Henrick K & Nakamura H. Announcing the worldwide protein data bank. Nature Structural and Molecular Biology. 2003;10(12):980.
CrossRef - Huey R., Morris G.M & Forli S. Using AutoDock 4 and AutoDock Vina with Auto Dock Tools A Tutorial. The Scripps Research Institute Molecular Graphics Laboratory. 2012.
- Weber L., Royal Soc Chemistry Thomas Graham House Science Park Milton Rd, Cambridge CB4 0WF, Cambs, England. 2008;5:65-66.
- Arnautova Y. A., Abagyan R. A & Totrov M. Development of a new physics‐based internal coordinate mechanics force field and its application to protein loop modeling. Proteins Structure, Function, and Bioinformatics. 2011;79(2):477-498.
CrossRef - Trott O & Olson A. J. Auto Dock Vina improving the speed and accuracy of docking with a new scoring function efficient optimization and multi threading. Journal of computational chemistry. 2010;31(2):455-461.
- Mangal M., Khan I ., M & Agarwal M. S. Acetogenins as potential anticancer agents. Anti-Cancer Agents in Medicinal Chemistry Formerly Current Medicinal Chemistry-Anti-Cancer Agents. 2016;16(2):138-159.
- Qayed W.S., Aboraia A. S., Abdel-Rahman H. M & Youssef A. F. Annonaceous acetogenins as a new anticancer agent. Der Pharma Chemica. 2015;7(6):24-35.
- Yang R.m., et al. Anticancer effect of total an no naceous ace togenins on hep a to carcinoma. Chinese journal of integrative medicine. 2015;2:682-688.
CrossRef







