Bahaa Al-Trad1, Mahmoud A. Al-Qudah2, Mazhar Al-Zoubi3, AlaaAl-Masri1, Riyadh Muhaidat1, Janti Qar1, Ghada Alomari1 and Nizar I. Alrabadi4
1Department of Biological Sciences, Yarmouk University, Irbid, Jordan.
2Department of Chemistry, Yarmouk University, Irbid, Jordan.
3Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid, Jordan.
4Department of Food Science and Nutrition, Faculty of Agriculture, Jerash University, Jerash, Jordan.
Corresponding Author E-mail: bahaa.tr@yu.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/1485
Abstract
Previous studies indicated that the extracts from different Ephedra species have antibacterial, antifungal and antioxidant activities. However, none of the published report described the phytochemical components and the antioxidant capacities of Ephedra alte belonging to the family Ephedraceae. To evaluate the in-vitro and in-vivo antioxidant activities of the butanolic extract from stems of Ephedra alte from northern Jordan. Graded concentrations of butanolic extracts from stems of E. alte plant were subjected to four different in-vitro antioxidant assays (DPPH, ABTS, ferrous ion chelating and hydroxyl radical scavenging activities). The in-vivo effects of two different doses of the extract (200 mg/kg and 500 mg/kg, orally for 12 days) on the activities of serum and liver superoxide dismutase (SOD) and catalase (CAT) were measured in mice. Strong in-vitro antioxidant activities in a concentration-dependent manner were recorded. As well, significant increases in both liver and serum CAT enzyme activity and in serum SOD activity were observed in mice treated for 12 days with the extract. These results suggested that the butanolic extract from stems of exhibited significant in-vitro and in-vivo antioxidant activities, supporting the use of E. alte as an important source of natural antioxidants.
Keywords
Antioxidant; Butanol Extract; Ephedra alte; In vitro; In vivo
Download this article as:| Copy the following to cite this article: Al-Trad B, Al-Qudah M. A, Al-Zoubi M, Al-Masri A, Muhaidat R, Qar J, Alomari G, Alrabadi N. I. In-Vitro and In-Vivo Antioxidant Activity of the Butanolic Extract from the Stem of Ephedra Alte. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Al-Trad B, Al-Qudah M. A, Al-Zoubi M, Al-Masri A, Muhaidat R, Qar J, Alomari G, Alrabadi N. I. In-Vitro and In-Vivo Antioxidant Activity of the Butanolic Extract from the Stem of Ephedra Alte. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22403 |
Introduction
Oxidative stress in animal cells reflects the imbalance between the production of antioxidants and oxidants which consequently leads to a severe damage of the cellular compartments and increased lipid peroxidation due to the action of reactive species.1,2 Oxidative stress has been interconnected to numerous chronic diseases.3 For instance, increasing evidence suggested a pathological impact of oxidative stress in the development of complications of the two major types of diabetes mellitus.1
The main reactive species include ROS and RNS, reactive oxygen species and reactive nitrogen species, respectively. ROS and RNS are generated in human body due to external and internal physiological processes. However, the imbalanced production of the oxidants can lead to the damage of many biomolecules (proteins, lipids, and nucleic acid).4,2 Normally, animal cells are equipped with many defense mechanisms against oxidative stress including glutathione (GSH), vitamins C and E, catalase (CAT), superoxide dismutase (SOD) and various peroxidases.4,2
Basically, antioxidants counteract the oxidation of biological molecules by delaying or inhibition mechanism.3 Early modulations of oxidative stress by exogenous natural antioxidants and diet rich in vitamins have proven a beneficial effect in the protection against the oxidative stress induced damage.2,5 Plant origin polyphenols; have gained considerable attention due to their possible health benefits. Epidemiological studies showed an effective impact of polyphenol plant diets on the reduction of the incidence of cancers, diabetes, osteoporosis, cardiovascular and neurodegenerative disorders.6
Jordanian traditional medicine included a list of more than 110 species from 49 plant families, mainly in the population of limited health care providers.7 Ephedra is a genus of the family Ephedraceae consistingof 50–65 species among which are shrubs, vines, but rarely small trees.8 Ephedra alte C. A. Mey (synonym Ephedra aphylla Forssk) is one of the common species in different Middle East countries.8 Results of previous studies on the biological activity of the plant indicted that the extracts from different Ephedra species have antibacterial, antifungal and antioxidant activities.9-11 However, there is no published report on the phytochemical composition and the antioxidant capacities of E. alte . Therefore, we aimed in this study to determine the total phenolic and total flavonoids of the butanolic extract from stem of E. alte that grows wild in northern Jordan and to determine its in vitro and in vivo antioxidant capacity.
Materials and Methods
Reagents and Plant Material
All reagents and chemicals were supplied and purchased from Sigma-Aldrich, USA unless otherwise specified. Ephedra alte was collected from the north of Jordan during spring of 2016. Plants material was identified by the plant taxonomist professor Ahmad El-oqlah from the Department of Biological Sciences, Yarmouk University.
Preparation of Crude Fraction
The fresh aerial parts were subjected to drying conditions at room temperature in a shady place for a month. Then the dried and powdered stems were subjected to extraction process using Soxhlet extractor with petroleum ether to remove the fatty acids, dried and then followed by methanol extraction. The rotary vacuum evaporator was applied for sample concentration and drying. This residue was partitioned between CHCl3 and H2O (1:1) solvent system. After the separation of CHCl3 and H2O phases, the dried CHCl3 fraction was partitioned between 10% aqueous methanol and hexane. The polar organic compounds were extracted from water by n-butanol.
Phytochemical Analysis
Crude fractions and distilled crude obtained from plants were screened for the presence of secondary metabolites of terpenes, saponins, flavonoids, tannins, alkaloids, anthraquinones, and cardiac glycosides following standard procedures described previously.12
Total Phenolic and Flavonoid Contents Analysis
Folin-Ciocalteu assay was used to evaluate and analyze the total phenolic contents as previously described.13 The results were expressed as mg/g gallic acid equivalent. The colorimetric aluminum chloride assay was used to evaluate and determine the total flavonoid content and expressed as mg/g quercetin.14
Antioxidant Activity In Vitro
DPPH Radical Scavenging Assay
The radical scavenging activity of the butanolic extract was determined by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay. Ascorbic acid (vitamin C) was used as a a positive control.15 Briefly, 1 mL of different concentration (5-500 μg/mL) of the extract was added to 2 mL of 0.1mM of DPPH/methanol solution, followed by 30 minutes incubation in dark conditions. The optical density was recorded at 517 nm against scavenger-free blank.
ABTS Assay
Antioxidant activity by 2,2’–Azino–bis (3-ethylbenzoline-6- sulfonic acid) diammonium salt (ABTS) decolonization assay was determined as previously described 15. The ABTS working solution was prepared by mixing equal quantities of 7 mM of ABTS and 2.4 mM of (K2S2O8) solutions and incubated at 2-3o C in dark conditions for 16 hours. The working solution was then diluted with d.H2O to obtain an absorbance of 0.75 ± 0.02 at 734 nm. The antioxidant assay reaction was performed by mixing 1 mL of the butanolic stem extract with 3mL ABTS working solution and incubated for 5 minutes. A serial concentrations of the extract were used (5-500 μg/mL) and the optical density was measured at 734 nm against the blank.
Ferrous Ion (Fe2+) Chelating Assay
Ferrous ions chelating activity was conducted as described earlier with slight modification.16 A 3 mL extract from each concentration (5-500 μg/mL) was added to 0.25 mL of 2 mM FeCl2 solution. A 0.2 mL of 5 mM ferrozine solution was added to initiate the reaction and left at room temperature for 10 min. EDTA solution was used as a positive control. The optical density was measured at 562 nm against the blank.
Hydroxyl Radical Assay
Salicylic acid was used to measure the hydroxyl radical formation according to the modified method of.16 A 1 mL of the butanolic extract solution from each concentration (5-500 μg/mL) was added to 250 μl of 6 mM FeSO4, followed by addition of 0.5 mL of 6 mM H2O2. The reaction mixture was to shaken and then allowed to stand for 10 min. Afterwards,a 1mL of 6 mM salicylic acid was added and incubated for 30 min at room temperature. Vitamin C was used as a positive control. The optical density was measured at 510 nm against the blank.
In Vivo Experiment
Acute Toxicity
The Institutional Ethics Committee at the Department of Biological Sciences, Yarmouk University approved all animal procedures and protocols. Different doses from the butanolic extract from E. alte stems were given to the mice (weighing 25-30 g; n=5/group) as follows: 50, 100, 200 mg/kg intraperitoneally (i.p) and 200, 500, 1000, 2000 mg/kg given orally. The mortilty and any sign of toxicity were observed regularly for the first 24 hrs and daily for 14 days.
Animal Treatment
Twenty-eight adult males Swiss albino mice, 8 weeks old and weighing ~25-30 g were maintained in the animal house unit at Yarmouk University under controlled conditions at 21 – 23°C on an illumination schedule of 12 hours of light. Standard pellet food and water were provided ad libitum. Mice were divided into three groups (n=7 in each group): Control and Ephedra alte extract treated groups (200 mg/kg and 500 mg/kg, orally for 12d). At the end of the experiments, the animals were weighed and anesthetized with ether, blood was collected, and the liver was excised rapidly and stored in liquid nitrogen.
In Vivo Antioxidant Activity
Serum was isolated from blood samples by centrifugation at 3000 rpm for 6 min at 4°C. Aklso, the liver was homogenized in phosphate buffer saline. After centrifugation at 15000 rpm for 15 min at 4°C, Serum and hepatic supernatants were used for oxidative stress assessment. CAT and SOD activities were measured using Amplite TM Fluorimetric Catalase Assay Kit (AAT Bioquest, USA) and SOD determination kit (Sigma–Aldrich, USA) following the manufacturer’s instructions.
Calculations and Statistical Analysis
In-vitro antioxidant activity data were recorded as means ± SEM of triplicate measurements. Scavenging or chelating effect (%) was calculated as the following: % = (control absorbance – sample absorbance/ control absorbance) × 100. The IC50 values were calculated by the linear regression method of plots of the percent of antioxidant activity against the concentration of the tested compounds. Statistical analyses of the in-vivo data were calculated using the SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL). P values were determined using one-way ANOVA followed by LSD. Differences were considered significant if P < 0.05.
Results and Discussion
The phytochemical screening in this study showed that the butanolic extract from the stem of E. alte is rich in tannins, flavonoids, saponins, alkaloids, and glycosides, supporting that E. alte may have medical benefit. It is well known that the Ephedra is a source of natural alkaloids products such as ephedrine that has been used medicinally to treat asthma, sinusitis and rhinitis.17,18 Additionally, pure isolated alkaloids are used as essential medicinal agents for their pain killer, antispasmodic and bactericidal effects.18
Oxidative stress is generated when the free radicals and oxidants are produced in excess which can damage many biological molecules that are important for cellular integrity and homeostasis.3 Oxidative stress is a primary cause of many disorders in humans such as neurodegenerative diseases, cancer and diabetes.19,1,3,6 Since scavenging of free radicals could inhibit the harmful effect of free radicals and stop the spreading of oxidation,4 antioxidants contents from plant origin through their scavenging activity are valuable for management of those diseases.6
Scientific evidence suggests that the flavonoids and phenolic acids, the most studied groups of polyphenols, play an essential role in protecting cell constituents against oxidative damage.6 In the present study, the butanolic extract from the stem of E. alte had a total phenolic of 404.001±5.53 mg/g gallic acid and flavonoids of 40.73±6.59 mg/g quercetin. In previous studies, the total phenolic content of E. procera was found to be of 718 mg tannic acid/g20 for E. sarcocarpa growing in Iran, 709.18 mg catechin equivalent/g extract10 for E. laristanica, 513 µmol gallic acid/g extract11 and for Ephedra strobilacea was 504.9 ± 41.51 μmol eq catechin/g extracts and 114.61 ± 15.13 μmol eq catechin/g extracts for the wild plants and callus, respectively.9 Recent studies showed that the flavonoids of E. alata growing in Palestine was in the range of 4.2 to 19.5 mg catechin/g and the phenolic content ranges from 30 to 101 mg gallic acid/g.14
In the current study, the in-vitro antioxidant activities of the butanolic extract from the stem of Ephedra alte were assessed against DPPH, ABTS and hydroxyl radicals. The ferrous ion chelating activity of the extracts was also determined. The butanolic extract showed different levels of radicals scavenging activity in a dose-dependent manner over the range of 5–500 μg/mL concentration (Table 1), indicating the high antioxidative capacity of the extract. The IC50, the concentration of the sample required to inhibit 50% of radical, of the extract were 66.4, 50.2, 43.5, 77.1 μg/mL for DPPH, ABTS, hydroxyl radicals and the ferrous ion chelating activity, respectively (Table 2).
Table 1: Antioxidant activity (%) of the butanolic extract from the stem of Ephedra alte.
| DPPH | ABTS | ferrous chelating | hydroxyl radical | |||||
| C(μg/ml) | BE | VC | BE | VC | BE | EDTA | BE | VC |
| 5 | 9.6±1.0 | 45.6±0.3 | 7.65±1.5 | 14.1±0.5 | 22.8±0.5 | 24.1±0.1 | 21.2±0.3 | 6.25±0.2 |
| 10 | 17.9±0.2 | 77.9±0.7 | 10.4±0.8 | 57.6±0.6 | 22.9±0.4 | 35.4±0.1 | 32.4±0.5 | 34.81±0.1 |
| 50 | 34.4±0.1 | 94.9±0.2 | 42.4±0.1 | 99±0.1 | 29.6±0.7 | 65.1±0.2 | 43.9±0.9 | 55.47±0.3 |
| 100 | 62.5±0.6 | 96.7±0.2 | 71.3±0.3 | 99.2±0.1 | 33.7±0.4 | 82.8±0.1 | 56.4±1.0 | 96.09±0.2 |
| 500 | 86.1±0.5 | 96.9±0.1 | 98.7±0.1 | 99.6±0.1 | 73.6±0.2 | 95.1±0.3 | 92.9±1.0 | 98.69±0.2 |
Data represent the mean ± SEM. Abbreviations: BE, butanolic extract; VC, vitamin c; DPPH, 2, 2-Diphenyl-1-picrylhydrazyl; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); EDTA, ethylenediaminetetraacetic acid.
Table 2: IC50 (μg/ml) of the butanolic extract from the stem of Ephedra alte.
| antioxidant activity | IC50 values (µg/ml) | |
| BE | VC | |
| DPPH | 66.4±0.55 | 1.6±0.03 |
| ABTS | 50.2±1.2 | 11.2 ± 0.45 |
| hydroxyl radical | 43.5±1.14 | 28.2±1.3 |
| BE | EDTA | |
| ferrous chelating | 77.1±1.1 | 21.8 ± 0.18 |
Data represent the mean ± SEM. Abbreviations: BE, butanolic extract; VC, vitamin c; DPPH, 2, 2-Diphenyl-1-picrylhydrazyl; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); EDTA, ethylenediaminetetraacetic acid.
Phytochemical components, including the phenolic and flavonoids, are important compounds that determine the plants antioxidant capacity, mainly due to their redox properties.21,5 The high antioxidant activity of Ephedra alte extract can be explained by the presence of the hydroxyl groups in the phenolic compounds.22 It has been shown previously that phenolic compounds provide the major contribution to the antioxidant activity of the methanolic extracts of Ephedra sarcocarpa measured by the DPPH assay.10 Therefore, the high phenolic constituents of the butanol extract of stem of Ephedra alte are responsible for its high antioxidative capacities.
Further confirmation of the antioxidant activity was conducted in vivo for the stem butanol extract. Biologically, the harmful effects of the ROS are defended by in vivo built-in mechanisms which involve enzymatic and non-enzymatic defense mechanisms. For instance, enzymatic antioxidant systems, CAT, GSH-Px, and SOD are the three important antioxidant enzymes which have an important role as a defense process that protects cells from the reactive oxygen species.2 Superoxide dismutase is one of the major mechanisms of defense against oxygen-derived free radicals, by converting superoxide radicals to H2O2, while CAT is a key enzyme of the enzymatic antioxidant systems which dismantling H2O2 to water and oxygen.2,23 Our study recorded a significant dose-dependent increase in CAT level in both liver homogenate and serum samples (P < 0.05; Fig 1 and 2) and in serum SOD level (P < 0.05; Fig 3) after 12d treatment with the stem butanol Ephedra alte extract in mice. However, no effect of the extract on hepatic SOD activity was observed (data not shown). Such data, coupled with the in vitro results indicated that the stem butanol extract of Ephedra alte could be an important source of natural compounds with antioxidant capacity.
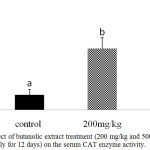 |
Figure 1: Effect of butanolic extract treatment (200 mg/kg and 500 mg/kg, orally for 12 days) on the serum CAT enzyme activity.
|
Data represent the mean ± SEM. Means with different superscript letters are significantly different from one another (P < 0.05).. Abbreviations: CAT, catalase.
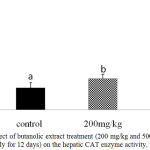 |
Figure 2: Effect of butanolic extract treatment (200 mg/kg and 500 mg/kg, orally for 12 days) on the hepatic CAT enzyme activity.
|
Data represent the mean ± SEM. Means with different superscript letters are significantly different from one another (P < 0.05).. Abbreviations: CAT, catalase.
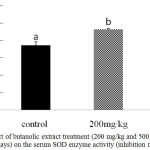 |
Figure 3: Effect of butanolic extract treatment (200 mg/kg and 500 mg/kg, orally for 12 days) on the serum SOD enzyme activity (inhibition rate %).
|
Data represent the mean ± SEM. Means with different superscript letters are significantly different from one another (P < 0.05). Abbreviations: SOD, superoxide dismutase.
Finally, acute oral toxicity test in the present study showed that the LD50 value of stem butanol extraction of E. alte was found to be more than 2000 mg/kg body weight for oral administration and more than 500mg/kg body weight for i.p administration. This indicates that butanol extraction from stems of E. alte might be non-toxic and safe when administered orally or i.p.
Conclusions
The butanolic extract from the stem of Ephedra alte showed high phenolic contents and exhibited high antioxidant activity both in vitro and in vivo which nominating the use of Ephedra alte as an important source for natural antioxidants.
Acknowledgments
We are very thankful to the Deanship of Research and Graduate Studies at Yarmouk University for their financial support under Grant Number 2/2017.
Competing Interests
The authors declare that they have no competing interests.
References
- Maritim A. C, Sanders A and Watkins J. B. Diabetes, oxidative stress and antioxidants a review. Journal of Biochemical and Molecular Toxicology. 2003;17:24-38.
CrossRef - Nimse S. B and Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Royal Society of Chemistry Advances. 2015;5:27986-28006.
CrossRef - Willcox J. K., Ash S. L and Catignani G. L. Antioxidants and prevention of chronic disease. Critical Reviews in food Science and Nutrition. 2004;44:275-295.
CrossRef - Kryston T. B., Georgiev A. B., Pissis P and Georgakilas A. G. Role of oxidative stress and DNA damage in human carcinogenes is. Mutation Research Fundamental and Molecular Mechanisms of Mutagenesis. 2011;711:193-201.
CrossRef - Rice-Evans C., Miller N and Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Science. 1997;2:152-159.
CrossRef - Pandey K. B and Rizvi S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid .Med Cell Longev. 2009;2:270-278.
CrossRef - Al-Khalil S. A survey of plants used in Jordanian traditional medicine. International Journal of Pharmacognosy. 1995;33:317-323.
CrossRef - Qasem J. R. Ephedra alte (joint pine): an invasive problematic weedy species in forestry and fruit tree orchards in Jordan. The Scientific World Journal. 2012;2012:10.
CrossRef - Parsaeimehr A., Sargsyan E and Javidnia K. A comparative study of the antibacterial, antifungal and antioxidant activity and total content of phenolic compounds of cell cultures and wild plants of three endemic species of Ephedra. Molecules. 2010;15:1668-1678.
CrossRef - Rustaiyan A., Javidnia K., Farjam M. H., Aboee-Mehrizi F and Ezzatzadeh E. Antimicrobial and antioxidant activity of the Ephedra sarcocarpa growing in Iran. Journal of Medicinal Plants Research. 2011; 5:4251-4255.
- Rustaiyan A., Javidnia K., Farjam M. H., Mohammadi M. K and Mohammadi N. Total phenols, antioxidant potential and antimicrobial activity of the methanolic extracts of Ephedra laristanica. Journal of Medicinal Plants Research. 2011;5:5713-5717.
- Siddiqui A and Ali M. Pratical Pharmaceutical Chemistry.1st Edition. CBS Publish and distributors. New Delhi. 1997:126-13.
- Singleton L., Orthofer R and Lamuela-Raventos R. Analysis of total phenols and other oxidation substrates and antioxidant by means of folin-ciocalteu reaget. Methods Enzymology. 1999;299:152-178.
CrossRef - Al-Rimawi F., Abu-Lafi S., Abbadi J., Alamarneh A. A., Sawahreh R. A and Odeh I. Analysis of phenolic and flavonoids of wild Ephedra alata plant extract by lc pda and lc ms and their anti-oxidant activity. African Journal of Traditional, Complementary and Alternative Medicines. 2017;14:130-141.
CrossRef - Al-Qudah M. A., Al-Ghoul A. M., Trawenh I. N., Al-Jaber H. I., Shboul A .T. M., Zarga A. M. H and orabi A. S. T. Antioxidant Activity and Chemical Composition of Essential Oils from Jordanian On on is Natrix L. and Ononis Sicula Guss. Journal of Biologically Active Products from Nature. 2014;4:52-61.
CrossRef - Mathew S and Abraham T. E. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2006;44:198–206.
CrossRef - Barker W. D and Antia U. A study of the use of Ephedra in the manufacture of methamphetamine. Forensic science international. 2007;166:102-109.
CrossRef - Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2:251–286.
CrossRef - Rhodes V . C. J., Moncol J., Izakovic M and Mazura M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological Interactions. 2006;160:1-40.
CrossRef - Dehkordi N. V., Kachouie M. A., Pirbalouti A. G., Malekpoor F and Rabei M. Total phenolic content, antioxidant and antibacterial activities of the extract of Ephedra procera fisch. et mey. Acta poloniae pharmaceutica. 2015;72:341-345.
- Labud J., Buckova M., Heilerova L., Silhar S and Stepanek I. Evaluation of the red ox properties and an tipro-oxidant effects of selected flavonoids by means of a DNA-based electro chemical biosensor. Analytical and bioanalytical chemistry. 2003;376:168-173.
CrossRef - Mathew S., Abraham T. E and Zakaria Z. A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol. 2015;52:5790-2798.
CrossRef - Winterbourn C. C. Superoxide as an intracellular radical sink. Free Radical Biology and Medicine. 1993;14:85-90.
CrossRef








