Arleni Bustami1,3, Popi Sopiah2, R. Muharam3 and Heri Wibowo1
1Integrated Laboratory Faculty of Medicine Universitas Indonesia.
2Biomedical Sciences Faculty of Medicine Unversitas Indonesia.
3Department of Obstetrics and Gynecology, Faculty of Medicine-RSCM Universitas Indonesia.
Corresponding Author E-mail: arleni.ab@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1514
Abstract
Endometriosis is a gynecologic disease in women that can cause infertility and chronic pelvic pain with a relatively high recurrence rate. This research was to prove the effects of gallic acid and its derivatives on inflammatory regulation of endometriosis primary cultures in terms of NF-kB mRNA expression and IL-6 secretions. Endometriosis cells are derived from endometriosis tissue of patients undergoing laparoscopy, isolated enzymatically and cultured primarily. The culture cells were treated with gallic acid, heptyl and octyl gallate at doses (25.6 μg/ml, 51.2 μg/ml and 102.4 μg/ml) for 48 h, then induced with 500 ng/ml LPS for 24 h. Inflammatory regulation was assessed from NF-kB mRNA expression with qRT-PCR and IL-6 secretion levels with ELISA. Gallic acid and its derivatives showed a decrease in the relative expression of NF-kB, significantly at dose 102,4 μg/ml. IL-6 although not statistically significant. The result indicated that gallic acid and its derivatives have a potential as anti-inflammatory effect. Gallic acid and its derivative compounds have an effect on decreased relative expression of mRNA NF-kB and IL-6.
Keywords
Endometriosis; Inflammation; NF-kB mRNA; IL-6
Download this article as:| Copy the following to cite this article: Bustami A, Sopiah P, Muharam R, Wibowo H. Effects of Gallic Acid and Its Derivates on Inflammatory Regulation of Endometriotic Primary Cultures: Study on NF-kB mRNA Expression and IL-6 Secretion. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Bustami A, Sopiah P, Muharam R, Wibowo H. Effects of Gallic Acid and Its Derivates on Inflammatory Regulation of Endometriotic Primary Cultures: Study on NF-kB mRNA Expression and IL-6 Secretion. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22447 |
Introduction
Endometriosis is a benign gynecological disorder characterized by the formation of mucous membrane-like tissue similar to the endometrium, grows outside the uterine cavity.1-2 Endometriosis is estimated to affect 6-10% of women of reproductive age. The incidence of endometriosis increases 25% -50% risk of infertility and 30% -50% of patients with endometriosis experience symptoms of pain and infertility which impact the quality of life of patients.2-5 Recurrence rates of post-surgical and hormonal treatment are still high, at 21.5% in the second year and 40-50% in the fifth year.5-6
The complex inflammatory process in the microenvironment becomes one of the causes affecting adhesion, invasion, proliferation, and apoptosis in endometriosis [6-8]. The two pathways involved in the inflammatory process that is the MAPK (mitogen-Activated Protein Kinase) and NF-kB (Nuclear Factor Kappa B). Activation of NF-kB transcription factor pathways affects the transformation of normal cells into endometrial cells, cell proliferation mediation, anti-apoptosis such as IL-6, pro-angiogenesis, and inflammation.9-12
Interleukin 6 (IL-6) is a cytokine that present in various inflammatory processes in endometriosis. IL-6 is known to increase the production of other cytokinesin endometriosis tissue.13-16 IL-6 plays a role in tumor growth and persistence of ectopic endometriosis tissue and inflammation in endometriosis. IL-6 found high levels in endometriosis.15 The up regulation of IL-6 cytokines happened through the activation of NF-kB pathway or MAPK in stromal cells of endometriosis.17
The involvement of NF-kB and IL-6 cytokines on the inflammatory mechanisms in endometriosis, provides an opportunity to find a new agent in the treatment of endometriosis through NF-kB pathway inhibition, one of them is gallic acid.
Gallic acid is a class trihydroxybenzoic acid of compounds 3,4,5-hydroxybenzoic acids and its derivate including heptyl and octyl gallate have the potential inhibit the spread of cancer cells, anti-oxidant, anti-mutagenic, and anti-inflammatory in some cell lines.18-20
Adding carbon atoms of gallic acid through esterification of the carboxyl groups produced alkyl ester derivative of gallates including heptyl and octyl gallate. The alkyl compounds are more hydrophobic than gallic acid, making them easier to penetrate through the cell wall.18
Gallic acid as an anti-inflammatory has been demonstrated (Murase, 1999) by inhibiting cytokine TNF-ɑ that play a role in the induction of translocation of NF-kB and decreased the expression endothelial-leukocyte adhesion molecule in cultured HUVEC (Human Umbilical Vein Endothelial Cell).20 This intracellular response clearly demonstrates that suppression of NF-kB activation by gallic acid can decrease the expression of genes that play a role in both inflammation and tumorogenesis but so far have not found the publication of the effects of heptyl gallate and octyl gallate as anti-inflammatory of endometriosis. So far, endometriosis treatment is aimed at relieving symptoms but has not been able to prevent recurrence and endometriosis survival. Development of target therapy with the aim of suppressing the growth of endometriosis tissue still needs further study. Gallic acid as one of the endometriosis therapy candidates has been proven effective as anti-cancer, anti-tumor, anti-inflammatory and some cell line in vitro, but has not proven its effectiveness in endometriosis cells.
In this study wanted to prove the effectiveness of gallic acid as a drug candidate against inflammatory regulation of endometriosis cells in vitro in terms of cell NF-kB mRNA expression and IL-6 levels.
Methods
This research is experimental in vitro and have passed the review of conduct by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia on January 4, 2016, no. 15/UN2.F1/ETIK/2016. This study used pellets and supernatant from primary culture of endometriosis cells. Endometriosis cells are derived from endometriosis tissue of patients undergoing laparoscopy, isolated enzymatically and cultured primarily. The culture cells were treated with gallic acid, heptyl and octyl gallate at doses (25.6 μg/mL, 51.2 μg/ml and 102.4 μg/ml) for 48 h, then induced with 500 ng/mL LPS for 24 h. Inflammatory regulation was assessed from NF-kB mRNA expression with qRT-PCR and IL-6 secretion levels with ELISA.
Isolation and Culture of Endometriosis Cells
Endometriosis cells were isolated from endometriosis tissue of Indonesian patients who had undergone laparoscopy, with enzymatic reactions using 0.2% collagenase type IV (Gibco, USA). Endometriosis cells were incubated at 37 °C 5% CO2, then cultured in DMEM F-12 (Gibco, USA) with 1% Pen-Strep, 1% Fungizone, 20% FBS (Gibco, USA).
Analysis of NF-kB mRNA expression with qRT-PCR
A number of 5×10 5 cells planted in well 12, then given gallic acid, heptyl gallate, and octyl gallate with concentration (25.6 μg/ml; 51.2 μg/ml; 102.4 μg/ml) for 48 h. Then induced 500 ng/ml LPS for 24 h. For positive control cells were induced by LPS only and cells without treatment for negative control. Pellets of endometriosis cells were carried out by RNA isolation of RNA mini kit Gene Science (Geneaid). Analysis of NF-kB mRNA expression used qRT-PCR with SBYR Fast Universal One Step qRT-PCR Kit (Kapabyosystem). ß-actin as housekeeping gene and the expression level of NF-kB was assessed by the Livak method.
IL6 Cytokine Secretions Assay
Supernatant cell cultures prepared for assay of IL-6 cytokines were derived from the culture medium which has been given the gallic acid , heptyl gallate, and octyl gallate with various concentrations. IL-6 secretions were assessed by ELISA kit (Quantikine, R&D, USA) according to protocol.
Results
Primary Culture of Endometriosis Cells
Endometriosis cell were isolated and cultured from three samples patients. Endometriosis cells growth show in fig.1.
 |
Figure 1: Morphology of primary endometriosis cells culture (inverted microscope, 200 x).
|
NF-kB mRNA Expression
Endometriosis cell cultures were treated with gallic acid, heptyl and octyl gallate for 48 hour later induced LPS for 24 h. NF-kB mRNA expression show in fig.2.
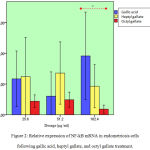 |
Figure 2: Relative expression of NF-kB mRNA in endometriosis cells following gallic acid, heptyl gallate, and octyl gallate treatment.
|
The relative mRNA expression of NF-kB in endometriosis cells with positive controls decreased compared to the relative expression of NF-kB with negative control assessed by q-RTPCR. There was no difference observed from NF-kB relative expression in gallic acid, heptyl, and octyl gallate at doses of 25.6 and 51.2 μg/ml. Significant statistic result of gallic acid, heptyl, and octyl gallate show mRNA NFkB suppression in a dose of 102.4 mg/mL (p value 0.049). The highest suppression was in the octyl group with a dose of 102.4 μg/mL. Suppression of mRNA NF-kB expression in octyl gallate greater than heptyl gallate and gallic acid.
IL-6 Secretions
After rationalization with negative control, levels of IL-6 decreased in cells were given gallic acid and octyl gallate compared to positive control, especially in octyl gallate dose 51.2 dose and 102.4 μg / mL. The effect of gallic acid and its derivatives to IL-6 secretions show in fig.3.
 |
Figure 3: Ratio of IL-6 secretion in endometriosis cells treated with gallic acid, heptyl and octyl gallate to IL-6 secretion in endometriosis cells without treatment and LPS stimulation.
|
Discussion
Gallic acid proved effective in several cell line as an anti-tumor by suppressing tumor cell viability, inhibiting proliferation, invasion and angiogenesis of tumors by inducing apoptosis also suppressing NFkB activation.21-24
The results of our study of gallic acid, heptyl gallate and octyl gallate indicate the ability of supression mRNA NF-kB, in line with earlier studies that gallic acid and synthetic derivatives of alkyl ester gallates effectively influence viability and cell proliferation in any type of cell line.16,25-27
Gallic acid is a candidate for antitumor and cancer agents with selective ability, the cytotoxic acid is cancerous but safe for normal cells. Weisburg (2014) found the gallic acid selective cytotoxic activity against oral cancer cells HSC-2 but not on ginggival fibroblast cells. Gallic acid induces apoptosis by increasing caspase 3 and cleavage of poly -ADP ribose polymerase causing morphological changes in cancer cells. The antioxidant properties of gallic acid are highly significant in biological activity thus increasing normal and cytotoxic cell protection against cancer cells.28 The gallic acid contained in the Solanum Surattense had antioxidant property also shows activity as free radical scavenging which is indispensable in the development of anti-oxidant therapy.29 This encourages the gallic acid as a potential anti-cancer agent that is safe and effective. Molecular docking results also show that gallic acid and its derivatives meet criteria of pharmacokinetic parameters to be developed as new drugs.30
Gallic acid has 1 H group on the carbon side chains with ClogP value 0.89, while heptyl gallate with the chemical formula -(CH2)6-CH3 with ClogP value 2.32, and octyl gallate (CH2)7-CH3 with ClogP value 3.7217 Heptyl and Octyl gallate is a synthetic derivative of gallic acid by adding an -OH group on the carbon side chains. The addition of OH groups to the group of gallic acid derivatives increases the solubility and hydrophobicity of the substance so as to facilitate the penetration and increase of the biological activity of the natural substances within the cell. Lipophilic chain length on the side chain alkyl ester form affect the affinity and cell membrane permeability to these substances.31-32 This supports the findings that heptyl and octyl gallate as an alkyl ester derivatives more potent as an agent blocking of NF-kB through suppression mRNA expression of endometriosis cell rather than pure gallic acid.
Suppression the relative expression of mRNA NF-kB with positive control was shown in groups conditioned by inflammation with LPS and the treatment resulted in relatively lower levels of expression than the ratio of untreated groups. The degree of relative suppression in the octyl group is higher than heptyl gallate and gallic acid. This proves the work of octyl gallate with more OH groups on carbon side chain is more potent in inhibiting NF-kB activity than heptyl gallate and gallic acid. The greater number of OH groups on the acidic carbon side chain increases the penetration ability into the cell, thus affecting the cell’s biological activity. These findings were parallel with previous studies which underlined that gallic acid was able to supress the expression of genes involved in inflammation and tumorogenesis.19
In this study, IL-6 level decreased when cells were given by octyl gallatte doses 51,2 and 102,4 compared to positive controls (p value = 0,004). The results show that octyl gallate may potentially suppress IL-6 levels compared to gallic acid and heptyl gallate. In the previous research by Soo-jin Jeong, et al (2016) the gallic acid contained in natural materials Radix Sanguisorbae give effect to decrease the levels of IL-6 on the cell line RAW 254.7. There was a reduction of activity Myeloperoksidase and p-STAT3 activity that cause supress mRNA IL-6, TNF-a, iNOS, and COX-2 expressions.33
Conclusions
The results of this study demonstrated that treatment of octyl gallate into endometriosis cells is capable of suppressing NFkB mRNA expression and IL-6 secretion. Octyl gallate has a more potential effects as an anti-inflammatory than gallic acid and heptyl gallate.
Acknowledgements
The funding of this research was supported by PUPT grant. The authors are thankful to dr. Herbert, SpOG for providing the necessary samples for this research.
Conflict of Interest
We declare there is no conflict of interest in this research.
References
- Ziegler D. D., Borghese B., Chapron C. Endometriosis and infertility : pathophysiology and management. Lancet. 2010;376(9742):730–8.
CrossRef - Carvalho L. F. P., Hui C., Celene Y., Ashok A. Endometriosis and infertility: biomarkers affecting implantation rate. ObstetGynecol. 2013;8(5):467–73.
CrossRef - Stilley J. A., Birt J. A., S-T K. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012;349(3):849–62.
CrossRef - Budi W., Puspita C. G., Sumapraja K., Natadisastra M., Harzief A. K., Situmorang H, et al. DLBS1442: Pilihan Penanganan Terkini pada Endometriosis. Medicinus. 2013;26:4–7.
- Macer M. L. Endometriosis and Infertility : A review of pathogenesis and treatment of endometriosis associated infertility. Obs Gynecol Clin N Am. 2012;39:535–49.
CrossRef - Stilley J. A., Birt J. A., Stilley J. A. W., Birt J. A., Sharpe-timms K. L. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012;349(3):849–62.
CrossRef - Verkauf B. Incidence symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc. 1987;74(9):671–5.
CrossRef - Jacquelin A. M., Hillary O. D. Inflammatory pathways in endometrial disorders. Mol Cel Endocrinol. 2011;3:41–51.
- Nickoloff B. J., Ben-neriah Ã. Y., Pikarsky E. Inflammation and cancer : is the link as simple as we think ? J Invest Dermatol. 2005;124(6):10–5.
- Gonzales R., Van L. A., Defrere S., Lousse J. C., Colette S., Devoto L. D. J. Involvement of the nuclear factor-kB pathway in the pathogenesis of endometriosis. Fertility and Sterility. 2010;94(6):1985–94.
CrossRef - Lousse J. C., Langendonckt A. V., Ramos R., Defrere S., Renkin E. D. J. Increased activation of nuclear factor-kappa B(NF-kB) in isolated peritoneal macrophages of patient with endometriosis. Fertility and Sterility. 2008;90(1):217–20.
CrossRef - Sakamoto Y., Harada T., Horie S., Iba Y., Taniguchi F. Y. S. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. 2003;88:730-5.
CrossRef - Richter O., Mallmann P., Van der ven H. K. D. TNF-alpha secretion by peritoneal macrophages in endometriosis. Zentralbl Gynakol. 1998;120(7):332–6.
- Malutan A. M., Drugan T., Costin N., Ciortea R., Bucuri C., Rada M. P., et al. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. 2015;40(6):96–102.
- Watanabe A., Terakawa N. Interleukin-10 attenuates TNF- a – induced interleukin-6 production in endometriotic stromal cells. Fertil Steril Internet. Elsevier Ltd. 2009;91(5):2185–92. Available from. http://dx.doi.org/10.1016/j.fertnstert.2008.04.052.
CrossRef - Ilie I., Ilie R. Cytokines and Endometriosis – the Role of Immunological Alterations. 2013;1(2):8–19.
- Loccatelli C., Filippin-Monteiro F. B. Alkyl esters of gallic acid as anticancer agents. Eur J Med Chem. 2013;60:233–9.
CrossRef - Jung H. J., Kim S. J., Jeon W. K., Kim B. C., Ahn K., Kim K., Kim Y. M., Park E. H. Anti-inflammatory activity of npropyl gallate through down regulation of NF-κB and JNK pathways. 2011;34(5):352–61.
- Verma S., Singh A. M. A. Gallic acid: Molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35:473–85.
CrossRef - Lu Y., Jiang F., Jiang H., Wu K., Zheng X., Cai Y., et al. Gallic Acid suppresses cell viability, proliferation, invasion, and angiogenesis in human glioma cells. Eur J Pharmacol. 2010;641(2-3):102–7.
CrossRef - Zhao B. H. M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol Lett. 2013;6(6):1749–55.
CrossRef - Kim N. S., Jeong S. I., Hwang B. S., Lee Y. E., Kang S. H., Lee H. C .O.C. Gallic Acid inhibits cell viability and induces apoptosis in human monocytic cell line U937. J Med Food. 2011;14(3):240–6.
CrossRef - Chao L. K., Kuang C. L., Yi S. M. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through action of NF-kB, Ras and matrix metalloproteinase-2 and -9. Oncol Rep. 2014;32:355–61.
CrossRef - Lima K. G., Krause G. C., Schuster A. D., Catarina A. V., Basso B. S., Mesquita D . F. C., et al. Gallic acid reduces cell growth by induction of apoptosis and reduction of IL-8 in HepG2 cells. Biomed Pharmacother. 2016;84:1282–90.
CrossRef - Nayeem N., Smb A., Salem H., Ahel-alfqy S. Gallic Acid A Promising Lead Molecule for Drug Development. 2016;8(2):8–11.
- Faried A., Kurnia D., Faried L. S., Usman N., Miyazaki T., Kato H., et al. Anticancer effects of gallic acid isolated from Indonesian herbal medicine Phaleria macrocarpa (Scheff) Boerl on human cancer cell lines. Int J Oncol. 2007;605–13.
CrossRef - Wang Q., Zhou K., Ning Y., Zhao G. Effect of the structure of gallic acid and its derivatives on their interaction with plant ferritin. Food Chem. Elsevier Ltd. 2016;213:260–7.
CrossRef - Weisburg J. H., Schuck A. G., Reiss S. E., Wolf B. J., Fertel S. R., Zuckerbraun H. L. B.H. Ellagic Acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells. Anticancer Res. 2013;33(5):1829–36.
- Yadav A., Bhardwaj R., Sharma R. A. Free Radical Scavenging Potential Of The Solanum Surattense Burm F: An Important Medicinal Plant. Int J Pharm Pharm Sci. 2014;6(3):39-42.
- Variya B. C., Modi S. J., Savani J. K. In-silico molecular docking and pharmacokinetic prediction of gallic acid derivatives as Ppar-Γ agonists. Int J Pharm Pharm Sci. 2016;9(1):2016–7.
CrossRef - Feng Q., Kumagai T., Nakamura Y., Uchida K., Osawa T. Correlation of antimutagenic activity and suppression of CYP1A with the lipophilicity of alkyl gallates and other phenolic compounds. Res. 2003;537:101–8.
CrossRef - Tammela P.., Laitinen L, Galkin A., Weennberg T., Heczko R., Vuorela H., et al. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch Biochem Biophys. 2004;425:193–9.
CrossRef - Chang S. S., Soo J. J., Sae R. Y., Na R. L. Quantitative Analysis and In vitro Anti-inflammatory Effects of Gallic Acid, Ellagic Acid and Quercetin from Radix Sanguisorbae. Pharmacogn Mag. 2016;12(46):104–8.
CrossRef








