Manuscript accepted on :January 10, 2018
Published online on: --
Plagiarism Check: Yes
Dania Mudar Shakir1, Shatha F. Abdullah2  and Inas K Sharquie2
and Inas K Sharquie2
1Department of Microbiology and Immunology, College of Medicine, University of Basrah, Basrah, Iraq.
2Department of Microbiology and Immunology, College of Medicine, University of Baghdad, Baghdad, Iraq.
Corresponding Author E-mail: shthabdullah@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1385
Abstract
Human herpesvirus 8 (HHV-8) is a common virus in the Mediterranean region. It has been linked to a number of malignancies, and it is believed to be the causative agent in certain cases. The aim of this study was to evaluate the distribution and possible association between HHV-8 and breast carcinoma, and to assess the risk factors associated with an HHV-8 infection. A total of 90 blood samples were collected from the study group. Forty-five of these patients were recently diagnosed with breast cancer and presented at the Oncology Centre in Basrah, Iraq, with ages ranging from 28 to 68 years old. Forty-five apparently healthy females matched for age and free from malignancy made up the control group, with ages ranging from 25 to 70 years old. The HHV-8 immunoglobulin G (IgG) antibody detection was done using an enzyme-linked immunosorbent assay with previously stored sera. HHV-8 was detected in 31.1% of the women with breast cancer, and a statistically significant difference was determined between the breast cancer patients and the control group. The highest HHV-8 seropositivity (17.8%) was seen in the 51–60 years old age group, and statistically significant differences were found between the patient and control groups with regard to the different age groups. Invasive ductal carcinoma was the most common type of breast malignancy in the women, with the majority of the patients classified as stage II. The histopathological types had a significant effect on the outcome proportion of the HHV-8 IgG antibodies. of the 14 breast cancer patients with blood transfusion histories, 6.7% were HHV-8 IgG antibody positive, indicating a significant difference with regard to the blood transfusion history. Diabetes mellitus was determined to be one risk factor associated with a high seropositivity of HHV-8, and it occurred at a rate of 13.3% among those women with breast cancer (p<0.05). In addition, it was found that the sexual route may be a significant risk factor. However, the HHV-8 infected breast cancer patients showed no statistically significant association with the coexistence of breast cancer markers. Based on the results of this study, female breast cancer may be associated with HHV-8. A blood transfusion history, diabetes mellitus and marriage were found to be risk factors for acquired HHV-8 infections in breast cancer patients.
Keywords
Breast Cancer Human Herpesvirus 8;
Download this article as:| Copy the following to cite this article: Shakir D. M, Abdullah S. F, Sharquie I. K. Serodiagnosis of Human Herpesvirus 8 in Women with Breast Cancer. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Shakir D. M, Abdullah S. F, Sharquie I. K. Serodiagnosis of Human Herpesvirus 8 in Women with Breast Cancer. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18910 |
Introduction
Several infectious viral agents exhibit significant associations with a variety of human cancers,1 with approximately 15–20% of all human cancers being caused by viruses.2 These oncogenic viruses appear to act at the beginning of tumour development or during the latent period on cells that already exhibit genetic dysregulation.3
Most seroepidemiological studies have shown a global human herpesvirus 8 (HHV-8) seroprevalence ranging from 2–10%, with a viral endemicity exhibiting geographical variation.4 HHV-8 has two modes of replication, latent and lytic stages5 and it seems to be under immunological control mechanisms producing no symptoms.4 The HHV-8 target cells are variable and numerous, including antigen presenting cells (dendritic cells, monocytes/macrophages and B lymphocytes),6 endothelial and spindle cells,7 epithelial cells,8 dorsal root sensory ganglion cells9 and keratinocytes.10 Several proteins have been reported to serve as HHV-8 entry receptors, which explains the wide cell tropism range.11
The viral genome encodes several protein homologues of the host cellular proteins, such as viral interleukin 6, chemokine-like molecules, interferon regulatory factors, complement binding proteins, viral cyclin D and viral Bcl2, which play major roles in the pathogenesis and progression of the related diseases.12
Materials and Methods
Study Population
A total of 90 blood samples were collected from breast cancer patients and healthy women after obtaining the approval of the ethics committee of the Ministry of Health. The patient study group was comprised of 45 females who were recently diagnosed with breast cancer, but not treated with chemotherapy, and who were undergoing consultation at the Oncology Centre in Basrah, Iraq. Their ages ranged from 28–68 years old. The control group was comprised of 45 apparently healthy females from the general population, including College of Medicine employees and medical personnel, and their ages ranged from 25–70 years old. They were matched for age and free from any type of cancer.
Blood Collection and Laboratory Investigations
Three to five millilitres of blood were collected aseptically from both the patients and healthy controls in sterile disposable plain tubes, and they were allowed to clot while standing oblique for one hour at room temperature. After centrifugation at 3,000 rpm for 5 minutes, the serum was separated and stored frozen at -20 C° until the serological examination was performed. The serological testing included the detection of anti-HHV-8 immunoglobulin G (IgG) antibodies via the anti-Kaposi’s sarcoma-associated herpesvirus/HHV-8 IgG enzyme-linked immunosorbent assay (ELISA) kit (catalogue no. 15-501-000; Advanced Biotechnologies Inc., Eldersburg, MD, USA), and the detection of cancer antigen 15-3 (CA15-3) via an ELISA kit (product code 5625-300; Monobind Inc., Lake Forest, CA, USA).
The hormone receptors [oestrogen receptor (ER) and progesterone receptor (PR)] and the human epidermal growth factor receptor 2 (HER2/neu) were detected via immunohistochemistry.
Results
The HHV-8 proportion was 31.1% among the women with breast cancer (Table 1), with a statistically significant difference (P<0.05). In addition, the serological results were variable among the different age groups (Figure 1). Moreover, the highest positivity of IgG antibodies was found in the 51–60 years old age group in the breast cancer patients (17.8%). Statistically significant differences were observed among the different age groups (P<0.05).
Ductal carcinoma was the predominant type of the breast cancer (Table 2), and it represented 37 (82.2%) out of the 45 total cases. All 14 of the HHV-8 IgG antibody positive cases exhibited ductal carcinoma (31.1%), and a significant difference was observed in relation to the histopathological types of breast cancer. With regard to the breast cancer stages, 8 (17.7%) of the women with breast cancer who had positive HHV-8 IgG results were in stage II (Figure 2).
Fourteen out of the 45 patients received blood transfusions, and 3 (6.7%) of them were positive for HHV-8 antibodies (Table 3). There was a significant difference among the patients with regard to their previous blood transfusion histories (P<0.05).
The percentage of positive HHV-8 IgG results was three times higher in the 6 diabetic breast cancer patients (13.3%), when compared with the 2 healthy diabetic women (4.4%), which was a significant difference. Thus, diabetes mellitus may be risk factor for an HHV-8 infection (odds ratio = 4.4) (Table 4).
The women were classified into two groups according to their marital status, as summarised in Table 5. Most of the women involved in this study were married (regardless of parity, divorced or widowed), for a total of 76 out of 90 women. All 14 of the women with breast cancer with HHV-8 seropositive results were married (31.1%). Therefore, the association between the marital status and HHV-8 antibodies was significant (P<0.05).
An analysis of the data was also done to study the association between HHV-8 and the breast cancer tumour markers (Table 6). With regard to tumour marker CA15-3, 1 patient (7.1%) was positive for HHV-8 antibodies. With regard to the PR, ER and HER2/neu, 9 out of the 45 patients were examined, and there were no cases positive for the HHV-8 IgG antibodies. Statistically, the differences between the HHV-8 IgG antibodies and the different tumour markers were not significant.
Table 1: Detection of the human herpesvirus 8 (HHV-8) immunoglobulin G (IgG) antibodies in the study groups.
| Study groups | HHV-8 IgG antibodies | Total
No. (%) |
|
| Positive (%) | Negative (%) | ||
| Breast cancer women | 14 (31.1) | 31 (68.9) | 45(100) |
| Healthy women | 5 (11.1) | 40 (88.9) | 45 (100) |
χ2 = 5.40 df = 1 P < 0.05
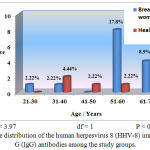 |
Figure 1: Age distribution of the human herpesvirus 8 (HHV-8) immunoglobulin G (IgG) antibodies among the study groups.
|
Table 2: Distribution of the human herpesvirus (HHV-8) immunoglobulin G (IgG) antibody results in relation to the histopathological types of breast cancer.
| Histopathological types | HHV -8 IgG antibodies response | Total No. (%) | |
| Positive No. (%) | Negative No. (%) | ||
| Ductal carcinoma | 14 (31.1) | 23 (51.1) | 37 (82.2) |
| Lobular carcinoma | —— | 4 (8.9) | 4 (8.9) |
| Tubular carcinoma | —— | 3 (6.7) | 3 (6.7) |
| Mucinous | —— | 1 (2.2) | 1 (2.2) |
| Total No. (%) | 14 (31.1) | 31 (68.9) | 45 (100) |
χ2 with Yates’ correction = 2.806 df = 1 P > 0.05
Fisher’s exact test P = 0.0436
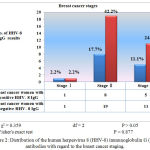 |
Figure 2: Distribution of the human herpesvirus 8 (HHV-8) immunoglobulin G (IgG) antibodies with regard to the breast cancer staging.
|
Table 3: Association between the human herpesvirus 8 (HHV-8) immunoglobulin G (IgG) antibodies and the blood transfusion history.
|
Study groups (Total No.) HHV -8 IgG |
Blood transfusion | Total
No. (%) |
||
| Received
No. (%) |
Not received
No. (%) |
|||
| Breast cancer women
(45) |
Positive | 3 (6.7) | 11 (24.4) | 14 (31.1) |
| Negative | 11 (24.4) | 20 (44.4) | 31 (68.9) | |
| Healthy women
(45) |
Positive | —— | 5 (11.1) | 5 (11.1) |
| Negative | 4 (8.9) | 36 (80) | 40 (88.9) | |
χ2 = 6.94 df = 1 P < 0.05 odds ratio = 0.7
Table 4: Detection of human herpesvirus 8 (HHV-8) immunoglobulin G (IgG) antibody results in relation to diabetes mellitus.
|
Study groups (Total No.) HHV- 8 IgG |
History of diabetes mellitus | Total
No. (%) |
||
| Diabetic women
No. (%) |
Not diabetic women
No. (%) |
|||
| Breast cancer women
(45) |
Positive | 6 (13.3) | 8 (17.8) | 14 (31.1) |
| Negative | 9 (20) | 22 (48.9) | 31 (68.9) | |
| Healthy women
(45) |
Positive | 2 (4.4) | 3 (6.7) | 5 (11.1) |
| Negative | 1 (2.2) | 39 (86.7) | 40 (88.9) | |
χ2 = 10 df = 1 P < 0.05 odds ratio = 4.4
Table 5: Relationship between the human herpesvirus 8 (HHV-8) immunoglobulin G (IgG) antibody results and the marital status.
|
Study groups (Total No.) HHV -8 IgG |
Marital status | Total
No. (%) |
||
| Married
No. (%) |
Unmarried
No. (%) |
|||
| Breast cancer women
(45) |
Positive | 14(31.1) | —— | 14 (31.1) |
| Negative | 30 (66.7) | 1 (2.2) | 31 (68.9) | |
| Healthy women
(45) |
Positive | 4 (8.9) | 1 (2.2) | 5 (11.1) |
| Negative | 28 (62.2) | 12 (26.7) | 40 (88.9) | |
χ2 = 12.18 df = 1 P < 0.05 odds ratio = 4.03
Table 6: Association between the human herpesvirus (HHV-8) immunoglobulin G (IgG) antibody results and the different breast tumour markers.
|
Tumour markers + / – |
HHV -8 IgG antibodies* | Total
No. (%) ** |
||
| Positive
No. (%) |
Negative
No. (%) |
|||
| 1- CA 15-3 | Positive | 1 (7.1) | 4 (12.9) | 45 (100) |
| Negative | 13 (92.9) | 27 (87.1) | ||
| 2- ER | Positive | —– | 2 (6.5) | 9 (20) |
| Negative | 1 (7.1) | 6 (19.4) | ||
| 3- PR | Positive | —– | 5 (16.1) | 9 (20) |
| Negative | 1 (7.1) | 3 (9.7) | ||
| 4- Her-2 neu | Positive | —– | 5 (16.1) | 9 (20) |
| Negative | 1 (7.1) | 3 (9.7) | ||
Fisher’s exact test P = 0.444 (PR and HER2/neu) P = 1 (ER and CA15-3)
* Positive HHV-8 IgG cases = 14, while negative HHV-8 IgG cases = 31.
** Patients may have had more than two positive tumour markers.
Discussion
Breast cancer is a major health burden worldwide, and it is the most common type of cancer in both developing and developed countries.13 Among Iraqi women, it represents the most frequently diagnosed malignant tumour.14 The oncogenic potential of HHV-8 makes it an important public health issue, because it poses unique mechanisms that influence cellular proliferation and the apoptotic control pathways.15
No previous studies have compared the prevalence of HHV-8 infections in breast cancer patients to healthy women in Iraq; therefore, this study presents preliminary data and provides a valuable base for redirecting upcoming studies in near future. The results show a significant association between positive HHV-8 IgG antibodies and female patients with breast cancer. This may be due to several factors, including the fact that HHV-8 is endemic to our region, the infection is acquired through nonsexual routes, there is a high number of travellers between the neighbouring countries (especially trips for religious purposes), and the different HHV-8 subtypes. These results are in agreement with a study carried out in Taiwan that reported HHV-8 DNA positivity in 45.2% of breast cancer patients.16
The seropositivity of HHV-8 is divergent with age-specific groups. In contrast to several epidemiological studies carried out in different regions that have documented high rates of HHV-8 with progressing age,17-19 this may be related to an uneven distribution across the population in various geographical areas, and that the paediatric age groups were excluded because all the participants were adult women. The study results are supported by a study carried out in Uganda showing a significant decline in the prevalence of HHV-8 with an increasing age among women.20 The present study shows a moderately significant association with regard to HHV-8 antibodies among the women with breast cancer in relation to age, which may be attributed to the disturbances in their immunological responses, in addition to the increased stress leading to the reactivation of silent HHV-8 infections or facilitating new HHV-8 infections.
The present study revealed a high percentage of ductal carcinoma when compared with the other types of breast cancer, and that most of the patients were in stage II. Similar results were obtained from the Iraqi national breast cancer research unit documents.21
The blood transfusion history exhibited a positive relationship with the HHV-8 serostatus among the immunocompromised recipients. One possible hypothesis is that in affected countries there exists an active viral circulation, acute infections with lytic viral replication, and higher blood viral loads. On the contrary, in non-endemic regions, healthy immunocompetent individuals who are HHV-8 seropositive may harbour predominantly latent HHV-8 infected cells that would not undergo lytic replication unless exposed to specific stimuli. Nevertheless, if both the lytic and latent cycles occur concurrently, the viral load may be low and frequently below the detection threshold. Moreover, there may be a lack of a leukodepletion protocol in the blood components in the blood bank strategy, unless it is indicated for certain at-risk patients.22,23 These results correspond to the numbers from previous studies from China, Argentina and Uganda.22-24
The current study did not focus on the type of diabetes mellitus, nor whether an HHV-8 infection precedes the occurrence of DM or causes the reactivation of a pre-existing virus. Statistically, it revealed that there was a strong link and that diabetes mellitus can be considered as a probable risk factor for HHV-8. The same evidence was observed in several previous studies.25-27 This can be explained by the fact that HHV-8 may be involved in the pathogenesis of diabetes mellitus via cytokine secretion, with subsequent induced inflammation mediated by natural killer cells or by direct infection of the pancreatic b cells.28 In addition, HHV-8 infections induce endoplasmic reticulum stress in the b cells, thus affecting insulin actions and creating secretion defects. Other mechanisms suggest that HHV-8 promotes interferon production and the upregulation of major histocompatibility complex class I molecules, leading to an autoimmune attack.28
Marriage is believed to be one marker of sexual activity and reproduction. The current study revealed a positive association between HHV-8 antibodies and the marital status. Similar findings were noticed in a number of previous studies.18,29,30 Conversely, one study carried out in male Zimbabwean factory workers showed that the prevalence of HHV-8 among their wives was nearly the same, regardless of the serostatus of the husband.31
The seropositivity rate of CA15-3 among the patients was 11.11% (5 out of 45 patients), in contrast to a previous study that found a high level of CA15-3 (75.9%) in patients with breast cancer.32 One explanation is that the majority of the women were in stage II with no distant metastasis, and CA15-3 is good indicator of cancer metastasis.33,34 Statistically, there were no associations between an HHV-8 infection and the tumour markers.
Acknowledgements
We thank the staff of the cancer center in Basrah for their help.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Source
This work is fully funded by researchers
References
- Blaho J.A, Aaronson S.A. Convicting a human tumor virus: Guilt by association? Proceeding of the national academy of sciences of the United States of America (PNAS). 1999;96(14):7619-7621.
CrossRef - Kalland K.H, Ke X.S, Oyan A.M. Tumour virology-history,status and future challenges. 2009;117(5-6):382- 399.
- Carbone M, Barbanti-Brodano G. Viral carcinogenesis. Oncology. 2006:214-232.
- Leao J.C, Porter S, Scully C. Human herpesvirus 8 and oral health care: an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(6):694-704.
CrossRef - Ma W, Galvin T.A, Ma H, Ma Y, Muller J, Khan A.S. Optimization of chemical induction conditions for human herpesvirus 8 (HHV-8) reactivation with 12-O-tetradecanoyl-phorbol-13-acetate (TPA)from latently-infected BC-3 cells. Biologicals. 2011;39(3):158-166.
CrossRef - Knowlton E.R, Lepone L.M, Li J, Rappocciolo G, Jenkins F.J, Rinaldo Ch R. Professional antigen presenting cells in human herpesvirus 8 infection. Frontiers in Immunology. 2012;3:427.
- 7-Moses A.V, Fish K.N, Ruhl R, Smith P.P, Strussenberg J.G, et al. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. Journal of Virology. 1999;73(8):6892-6902.
- Grange P.h.A, Marcelin A.G, Calvez V, Chauvel C, Escande J.P, Dupin N. Salivary Lactoferrin Is Recognized by the Human Herpesvirus-8. Journal of Investigative Dermatology. 2005;124:1249–1258.
CrossRef - Corbellino M, Parravicini C, Aubin J.T, Berti E . Kaposi’s sarcoma and herpesvirus-like DNA sequences in sensory ganglia. NEJM. 1996;334(20):1341-1342.
CrossRef - Wang F.Z, Akula S.M, Sharma-Walia N, Zeng L, Chandran B. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. Journal of Virology. 2003;77(5):3131-3147.
CrossRef - Rappocciolo1G, Hensler H.R, Jais M, Reinhart T.A, Pegu A, Jenkins F.J, Rinaldo Ch R. Human Herpesvirus 8 Infects and Replicates in Primary Cultures of Activated B Lymphocytes through DC-SIGN. Journal of Virology. 2008;82(10):4793-4806.
CrossRef - Murphy M.P, Khoi Ha N, Xiang Ch, Chen Y, Gillim L , et al. Transcription Program of Human Herpesvirus 8 (Kaposi’s Sarcoma-Associated Herpesvirus). Journal of Virology. 2001;75(10):4843-4853.
CrossRef - Bray F, McCarron P, Parkin D.M. The changing global patterns of female breast cancer incidence and mortality. Breast cancer research. 2004;6(6):229-239.
CrossRef - Alwan N. Iraqi initiative of a regional comparative breast cancer research project in the Middle East. Journal of Cancer Biology & Research. 2014;2(1):1016.
- Sgarbanti M, Arguello M, TenOever B.R, Battistini A, Lin R, Hiscott J. A requirement for NF-B induction in the production of replication-competent HHV-8 virions. Oncogene. 2004;23:5770-5780.
CrossRef - Tsai J.H, Tsai C.H, Cheng M.H, Lin S.J, Xu F.L, Yang C . Association of viral factors with non-familial breast cancer in Taiwan by comparison with noncancerous; fibroadenoma; and thyroid tumor tissues. Journal of medical virology. 2005;75:276-281.
CrossRef - Edoardo P. Human Herpesvirus-8 and other viral infections, Papua New Guinea. Emerging Infectious Diseases. 2001.
- Shebl F.M, Sh D.C, Pfeiffer R.M, Biryahwaho B, Amin M.M, et al.Human Herpesvirus 8 Seropositivity Among Sexually Active Adults in Uganda. PLoS One. 2011;6(6):21286.
CrossRef - He F, Wang X, He B, Feng Z, Lu X, et al . Human herpesvirus 8: serovprevalence and correlates in tumor patients from Xinjiang. China.J Med Virol. 2007;79(2):161-166.
CrossRef - Wawer M.J, Eng S.M, Serwadda D, Sewankambo N.K, Kiwanuka N, Chuanjun L, Gray R.H . Prevalence of Kaposi Sarcoma-Associated Herpesvirus compared with selected sexually transmitted diseases in adolescents and young adults in rural Rakai district, Uganda. STD. 2001;28(2):77-81.
CrossRef - Elyass T.Y. Molecular study of Human Mammary Tumor virus and immunohistochemistry of hormonal receptors in women with breast carcinomas. 2012. MSc. thesis, College of Medicine, Baghdad.
- Wang X, He B, Zhang Z, Liu T, Wang H, et al. Human herpesvirus-8 in north western China: epidemiology and characterization among blood donors. Virology Journal. 2010;7:62.
CrossRef - Sosa C, Benetucci J, Hanna C, Sieczkowski L, Deluchi G, et al. Human herpesvirus8 can be transmitted through blood. Medicina(Buenos Aires). 2001;61(3):291-294.
- Hladik W, Dollard S.C, Mermin J, Fowlkes A.L, Downing R, et al. Transmission of human herpesvirus 8 by blood transfusion. NEJM. 2006;355(13):1331-1338.
CrossRef - Ingianni A, Carta F, Reina A, Manai M, Desogus A, Pompei R. Prevalence of Herpesvirus 8 infection in type 2 diabetes mellitus patients. American Journal of Infectiuos Diseases. 2007;3(3):123-127.
CrossRef - Sobngwi E, Choukem S.P, Agbalika F, Blondeau B, Fetita L.S, et al. Ketosis-prone type 2 diabetes mellitus and Human Herpesvirus 8 infection in Sub-Saharan Africas. JAMA. 2008;299(23):2770-2776.
CrossRef - Caselli E, Rizzo R, Ingianni A, Contini P, Pompei R, DiLuca D. High prevalence of HHV8 infection and specific killer cell immunoblobulin-like receptors allotypes in Sardinian patients with type 2 diabetes mellitus. JMV. 2013.
- Christophe M, Filippi G, Mathias G, Herrath V. Viral trigger for type 1 diabetes pros and cons. Diabetes. 2008; 57(11) :2863-2871.
CrossRef - Mbulaiteye S.M, Pfeiffer R.M, Dolan B, Tsang V.C.W, Noh J, et al. Seroprevalence and risk factors for Human Herpesvirus8 infection, rural Egypt. Emerging infectious diseases. 2008;14(4):586-591 .
CrossRef - Wojciki J.M, Newton R, Urban M.I, Stein L, Hale M, et al. Risk factors for high anti-HHV-8 antibody titers(≥ 1:51,200) in black,HIV-1 negative South African cancer patients: a case control study. BMC infectious diseases. 2003;3:21-32.
CrossRef - Campbell T.B, Borok M, Ndemera B, Fiorillo S, White I.T, et al. Lack of evidence for frequent heterosexual transmission of Human Herpesvirus 8 in Zimbabwe. Clin Infect Dis. 2009;48(11):1601-1608.
CrossRef - Cao R, Wang L.P. Serological diagnosis of liver metastasis in patients with breast cancer. Cancer BIOL Med. 2012;9:57-62.
- Shering S.G, Sherry F, McDermott E.W, O’Higgins N.J, Duffy M.J. Preoperative CA15-3 concentrations predict outcome of patients with breast carcinoma. Cancer. 1998;83(12):2521-2527.
CrossRef - Yerushalmi R, Tyldesley S, Kennecke H, Speers C, Woods R, Knight B, Gelmon K.A. Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome. Annals of oncology. 2012;23(2):338-345.
CrossRef







