Manuscript accepted on :January 30, 2018
Published online on: --
Plagiarism Check: Yes
Khalissa Deffar1 , Khenchouche Abdelhalim2
, Khenchouche Abdelhalim2 , Xing Xie3
, Xing Xie3 , Li Ying3
, Li Ying3 , Soraya Ouhida4 and Abbes Mahnane5
, Soraya Ouhida4 and Abbes Mahnane5
1Department of Biochemistry, School of Nature and Life Sciences, Ferhat Abbas University Sétif-1-, Sétif, Algeria.
2Department of Microbiology, School of Nature and Life Sciences, Ferhat Abbas University Sétif-1-, Sétif, Algeria.
3Department of Gynecologic Oncology, Zhejiang University, Hangzhou, Zhejiang Province, China.
4Department of Pathological Anatomy, University Hospital Center, Sétif, Algeria.
5Department of Medicine, Ferhat Abbas University Sétif-1-, Sétif, Algeria.
Corresponding Author E-mail: khalissa_deffar@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1348
Abstract
The Objective of the present study is to evaluate the expression levels of Bcl-2 and p53 proteins in squamous cell carcinoma and adenocarcinoma of the uterine cervix, and try to explain their role as prognostic markers for this cancer. The cohort comprised 90 cases of the cervix lesions. The samples were assessed by immunohistochemistry for the expression of Bcl-2 and p53 proteins. The results showed that the Bcl-2 expression was either absent, low or moderate respectively in 38.96%; 50.65% and 10.39% of SCC cases. However, it was absent or expressed in 76.92% and 23.08% of adenocarcinoma cases respectively. The p53 protein was absent or present respectively in 75.32% and 24.68% of SCC cases as demonstrated by immunohistochemistry. p53 was almost absent in adenocarcinoma samples where only 7.70% of cases were positive. There was no significant correlation between Bcl-2 and p53 expression (p=0.352). We conclude that p53 expression, detected by immunohistochemistry, does not appear to be a prognostic marker for cervical cancer. Nevertheless, Bcl-2 expression seems to provide more information for this disease. It may represent an important indicator for cervical cancer.
Keywords
Adenocarcinoma; Bcl-2; Cervix Cancer; Prognosis p53; Squamous Cell Carcinoma;
Download this article as:| Copy the following to cite this article: Deffar K, Khenchouche A, Xing X, Ying L, Ouhida S, Mahnane A. Immunohistochemical Expression of p53 and Bcl-2 in Algerian Cervical Carcinoma. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Deffar K, Khenchouche A, Xing X, Ying L, Ouhida S, Mahnane A. Immunohistochemical Expression of p53 and Bcl-2 in Algerian Cervical Carcinoma. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18901 |
Introduction
Cervical cancer is the fourth most common cancer in women worldwide with an estimated one-half million of new cases in 2012. A large majority of the global burden occurs in developing countries. There were an estimated 266.000 deaths from this cancer worldwide in the same year. In Algeria, cervical cancer is the third most common cancer in women, with an incidence of 1,288 cases and 510 deaths estimated in 2012.1 The uterine cervix carcinoma includes both slowly and rapidly progressing tumours. The alteration in apoptosis disturbance, in immune surveillance, uncontrolled proliferation caused by increased of cell growth and/or loss of growth suppression are the characteristics of cervical cancer progression.2
Almost all cervical cancers are caused by the infection with high-risk human papillomaviruses (HR-HPV). In this case, the viral DNA integration mainly occurs into the human chromosomal DNA. The tumour suppressor genes are deactivated by viral oncoproteines, or the proto-oncogenes are activated to oncogenes. This emphases the rates of cell proliferation and leading to cervical intraepithelial neoplasia (CIN).3 Authors insist that the persistence of infection by HR-HPV is the best predictor of increased risk of cervical cancer but others consider that the over-expression of several other markers of cell proliferation and apoptosis such as p53, Bcl-2, p16, Ki-67 are also important.4,5
The Bcl-2 gene family seems to act as a regulatory of the apoptotic pathway. In this family, Bcl-2 and Bax are most important apoptosis regulating proteins. Bcl-2 is a member of the anti-apoptotic sub-family and Bax is a member of the pro-apoptotic sub-family.6 Originally, the Bcl-2 gene was detected in B-cell lymphomas bearing a chromosomal translocation t(14, 18).7 The Bcl-2 protein is localized at the mitochondrial outer membrane, in the nuclear envelope, plasma membrane and endoplasmic reticulum.8,9 The over-expression of Bcl-2 protein looks like to be associated with the blocking of apoptosis.10 The expression of Bcl-2 and the formation of hetero and homo dimmers between members of the Bcl-2 family are important for the ability of Bcl-2 to inhibit programmed cell death.11 Liang et al. claimed, by in vitro experiments, that increased Bcl-2 expression under conditions of p53 inactivation might provide cervical carcinomas cells with a selective growth advantages. This finding could imply that Bcl-2 expression may play an important role in the genesis and progression of cervical cancer.12
The p53 protein has 53 kDa in its molecular weight, it is encoded by p53 gene located on chromosome 17 that is involved in many cellular processes such as cell cycle control, DNA repair, initiation of apoptosis and control of cell differentiation.13,14 The p53 is a transcriptional regulator, with a short half life time of several minutes; it both activates and represses several genes.13,15,16,17,18,19 The rise in p53 protein level lead to changes in expression of p53 responsive genes in response to cellular stress, particularly DNA damage, which allow a subsequent cell cycle arrest and/or cell death. Growth arrest or apoptosis prevents damaged DNA from being replicated and suggests a role of p53 in preserving the integrity of the genome. In some cells and in addition of these role, p53 can induce apoptosis and can down-regulate Bcl-2.13,20
On the other hand, the inactivation of p53 in certain cancers is usually the result of a point mutation in exons 5-8 leading to an amino acid change in the p53 protein which will lose the ability to turn off the cell cycle and show a great genomic instability.21,22 Not all human cancers associated with p53 protein have a high frequency of point mutations.23 The mutated gene product of this mutation can be detected by immunohistochemistry, which demonstrated a longer half-life than the wild type protein and increased genetic instability.24 The mutations are rare in cervical cancers which are associated mainly with HPV infection.25,26 Molecular biology data on the natural history of this infection and cervical cancer suggest that viral infections interfere with the mechanism of cellular growth, DNA repair and immunologic responses. Inactivation of p53 protein by E6 oncoprotein of HPV has been proven.27 The contradictory reports make the association between Bcl-2 and p53 expression of cervical cancer not well-understood.5,28,29
The purpose of this study is to evaluate the expression of p53 and Bcl-2 in Algerian cervical carcinoma samples in an attempt to explain their prognosis value using the immunohistochemistry detection.
Materials and Methods
Tissue Samples
Ninety cases of squamous cell carcinomas and adenocarcinomas of the uterine cervix were used in this cohort. All the samples were collected from the pathology laboratory of Sétif’s Hospital. The patient’s mean age of diagnosis was 51 years (SD ± 10.18). All these samples were HPV 16 and/or 18 positive. The nature of specimens was cervical biopsies embedded in formalin paraffin.
Immunohistochemistry
Paraffin blocks of these patients were retrieved and three sections of 3-5 µm thickness were taken on Poly L lysine coated slides. The first group of slides were stained with routine Hematoxylin and Eosin coloration. The two other groups of slides were stained by the use of monoclonal mouse anti-human Bcl-2 oncoprotein (clone 124 ; code M0887 antibody, Dako) and monoclonal mouse anti-human p53 protein (clone DO-7; code M7001, Dako) antibodies. These antibodies were used separately. The Bcl-2 and p53 immunohistochemistry was carried out on sections tissues using Dako REALTMENVisionTMDetection System, Peroxidase/DAB, Rabbit/Mouse. This system offers the Dako REALTM DAB+ Chromogen to visualize the reaction. The monoclonal mouse anti human Bcl-2 oncoprotein and monoclonal mouse anti-human p53 protein were used at the dilution of 1:50 when applied on paraffin-embedded sections of cervical cancer.
Bcl-2 staining gave brown cytoplasmic reactivity. A case was taken as positive if more than 10% cells show cytoplasmic reactivity.30 The staining of p53 gave brown nuclear reactivity. A case was considered positive if more than 5% nuclei were stained.31
Statistical Analysis
The SPSS 20 and Excel 2013 software have been used for the statistical analyses. Correlation between variables (the percentage of both Bcl-2 expression and p53 expression) was determined by Pearson’s coefficient, p value of 0.05 or less was considered statistically significant. The bivariate regression analysis was performed on the percentage of both Bcl-2 expression and p53 expression. The crude and adjusted odds ratios (ORs [95% CI]) for the negative and positive Bcl-2 and p53 expression were also reported.
Results
Immunohistochemical Analysis of Protein Expression
The immunostaining evaluation of all slides was done. A Bcl-2 staining was semi quantified as negative (˂ 5% positive cells), weak (5 to 50% positive cells), moderate (50 to 90% positive cells) or strong (˃ 90% positive cells).32 The p53 staining was scored in two categories ˂ 5% and ≥ 5% of the cells being p53 positive.2 The Bcl-2 expression was always diffusely localized in the cytoplasm of the cells and was present in 47 of squamous cell carcinoma (61.04%). However, within the same tumour, the expression of Bcl-2 was heterogeneous and it could sometimes vary from strongly positive to negative (Figure 1). The immuoreactivity of p53 was nuclear in the neoplastic cells and was present in 19 cases of squamous cell carcinoma (24.68%). Sometimes cytoplasmic staining of p53 was seemed in dispersion. The analyses are based on the results of nuclear staining only (Figure 2). The photomicroscopy of the slides were taken in different magnifications (x50 ; x100 ; x200 and x400) using an optic microscope connected with a digital camera (Leica, Germany). The results of Bcl-2 and p53 staining are resumed in table 1 and table 2.
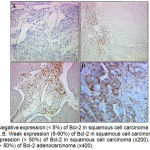 |
Figure 1: A. Negative expression (˂ 5%) of Bcl-2 in squamous cell carcinoma of the uterine cervix (x200). B. Weak expression (5-50%) of Bcl-2 in squamous cell carcinoma (x200). C. Moderate expression (˃ 50%) of Bcl-2 in squamous cell carcinoma (x200). D. Moderate expression (˃ 50%) of Bcl-2 adenocarcinoma (x400).
|
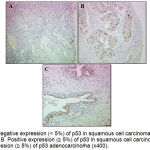 |
Figure 2: A. Negative expression (˂ 5%) of p53 in squamous cell carcinoma of the uterine cervix (x200). B. Positive expression (≥ 5%) of p53 in squamous cell carcinoma (x200). C. Positive expression (≥ 5%) of p53 adenocarcinoma (x400).
|
Table 1: Staining results of Bcl-2 protein
| ˂ 5%, n (%) | 5-50%, n (%) | > 50%, n (%) | Total, N | |
| Squamous cell carcinoma | 30 (38.96) | 39 (50.65) | 8 (10.39) | 77 |
| Adenocarcinoma | 10 (76.92) | 3 (23.08) | 0 (0) | 13 |
| 90 |
Table 2: Staining results of p53 protein
| ˂ 5%, n (%) | ≥ 5%, n (%) | Total, N | |
| Squamous cell carcinoma | 58 (75.32) | 19 (24,68) | 77 |
| Adenocarcinoma | 12 (92.30) | 1 (7.70) | 13 |
| 90 |
(n: number of cases)
The Bcl-2 is more expressed in squamous cell carcinoma than in adenocarcinoma of the uterine cervix (ORs= 5.55, 95% CI). The correlation between p53 and Bcl-2 expression, determined in the 77 cases of the squamous cell carcinoma, was poor for p value of 0.352. That shows the independence of two proteins expression (Figure 3).
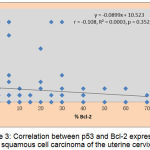 |
Figure 3: Correlation between p53 and Bcl-2 expression in squamous cell carcinoma of the uterine cervix.
|
Discussion
In our work, in all cases of squamous cell carcinomas and adenocarcinomas of uterine cervix, Bcl-2 protein was more demonstrated than p53 protein. This could be due to p53 degradation by ubiquitination E6-HPV mediated. We demonstrated negative and positive expression of p53 protein by immunohistochemistry in 75.32 (58/77) and 24.68% (19/77) of SCC cases respectively. For the adenocarcinoma samples, almost cases showed negative expression (92.30%, 12/13) only 7.70% (1/13) of cases were positive. The tumours studied showed an occasional focus of p53 immunoreactive cells where cells exhibited less than 5% of p53 positivity, by the use of an antibody which detects not only mutated but also wild type p53 protein. In cervical cancer, the p53 immunohistochemistry results have been somewhat confusing. The half-life time of wild type p53 protein is very short, and it cannot be detected by immunohistochemistry.33,34 Moreover, this technique is considered not sufficiently sensitive as compared with DNA detection. However, over-expression or mutated p53 protein have a long half-life and can recognized by immunohistochemistry. Mutated p53 protein complexed with other proteins, such as heat shock protein may not be completely degraded by the proteases system involving the gene E6 products of HPV and can be detected by immunohistochemistry.35,36
Several studies have evaluated p53 expression in cervical cancer. Some of whom also included cases under follow-up for genital HPV infection.5,28,37 Many studies have showed difference rates of p53 expression. High positivity rates ranging between 60% and 80% were found by many authors.38,39 Other work showed the carcinomas cervix exhibited 50% of p53 positivity.40 However many authors have found lower positivity rates ranging between 10 and 50%41,42,43 which we have also found in our study but only in few cases of cervical cancer. A study done by Dimitrakakis et al. (2000), to assess the expression and clinical significance of Bcl-2 and p53 in the progression of cervical cancer, showed that despite the expression of p53 was higher in invasive SCC than in adenocarcinomas but there was no significant correlation between the expression of p53 and the histological type of cervical carcinoma.29
The different percentage of p53 expression observed in literature could be explained by the difference in the used protocols, the type of antibody used that recognizes wild type and mutant p53, and different scoring cut-off of the protein expression. Normal cervical epithelium adjacent to tumour did not stain p53 protein, which confirms reports by Cattoretti et al. and Caamano et al. that the state level of normal p53 protein is too low to be detected by immunohistochemistry.33,44
The deregulation of p53 function, in cervical cancer, through direct neutralization of the protein by E6 produced by HPV types 16 and 18, has been proven.45,46 Elimination of p53 protein by E6 is an important step in tumorogenesis of HPV-associated cervical cancer.47
Over-expression of p53 protein would not be found to occur in HPV 16-18 associated tumours where E6 binds and degrades the p53 protein. The p53 mutation could only play a role in the tumorogenesis of HPV 16-18 negative cervical cancer. Some studies showed that p53 protein accumulation may be an early event in carcinogenesis, while for others, p53 over-expression or mutation did not seem to play a significant role in cervical tumorogenesis.48,49,50 A study of Chen et al. suggested that expression of p53 might be indicative of an unfavourable prognosis in patients with squamous cell carcinoma of the uterine cervix.41
In this study, we found a negative, low and moderate expression of Bcl-2 protein in 38.96% (30/77), 50.65% (39/77) and 10.39 (8/77) of SCC cases respectively, and negative or low expression in respectively 76.92% (10/13) and 23.08 (3/13) of adenocarcinoma cases. Several studies evaluated the Bcl-2 expression in cervical cancer. Almost of these studies found the increased expression in the advanced stages of cervical cancer40,51,52 contrary to that observed by Tjalma et al.32
During the last years, the significance of the apoptotic pathway in the development and progression of human malignant tumours has become an important subject of discussion. Bcl-2 is one of the proteins that have a regulating role in the programmed cell death. Bcl-2 prevents cells from apoptosis and it is a strong independent prognostic parameter.32 It is commonly clear that prolonged cell survival with inhibition of apoptosis is associated with carcinogenesis. Earlier Tjalma et al. (2001), found that the expression of Bcl-2 is a strong prognostic parameter because this expression is lost during tumour progression and, suggesting that the regulating of apoptosis plays a critical role in the behaviour of cervical carcinomas.2 Otherwise Shukla et al. (2014), used premalignant and malignant lesion of uterine cervix in their study, showed no significant association between HPV 16/18 infection and p53 and Bcl-2 expression. Although Bcl-2 staining showed a significant difference between CIN and carcinoma cervix. They conclude that Bcl-2 immunostaining may be used as a diagnostic biomarker to differentiate malignant lesions from the premalignant lesions.40 Dimitrakakis et al. showed that Bcl-2 expression was increased in relation with the grade of CIN. In addition, a significant difference between the expression of Bcl-2 in premalignant lesions and in carcinomas was found in the same study.29
Grace et al. showed an abnormal cytoplasmic expression of bcl-2 protein and nuclear expression of p53 protein were observed using immunocytochemistry in biopsies of cervical lesions but not in normal samples. The intensity of immunoreactivity for both p53 and Bcl-2 proteins varied between different grades of cervical lesions. By this study, it suggested that the immunodetection of both p53 and Bcl-2 proteins in squamous cell carcinoma of the uterine cervix can be used as an independent diagnostic biomarker for cervical cancer associated with HPV infection. The highly significant association of these proteins with HPV infection suggests that the HR-HPV infection may be responsible for the co-overexpression of p53 and Bcl-2 in cervical cancer even though both of them are antagonistic in their function.28 In breast cancer, there is a negative correlation between p53 accumulation and Bcl-2 expression.53 In some cells, p53 can induce apoptosis and can down-regulate Bcl-2.13,20 In this study, the p value was 0.352 means that the correlation between the expression of Bcl-2 and p53 protein is not significant.
Conclusion
The evaluation of Bcl-2 expression may provide additional prognostic information for the cervical cancer and therefore to be developed as a prognostic biomarker for this disease. In cervical cancer, the results of p53 immunohistochemistry have been somewhat confusing. and does not appear to be a prognostic parameter for this type of cancer. Among different cancers, the Bcl-2 and p53 pathway leading to programmed cell death may not be regulated in the same way. It has been demonstrated in vitro that the absence of functional p53 in the cervical cells it has increased Bcl-2 expression. This increased expression under conditions of p53 inactivation may provide cells with a selective advantage for cell survival and consequently play a role in the development of cervical tumorigenesis.
Acknowledgment
We thank the pathology division members, Sétif Hospital, Algeria, for their help to collect the samples.
We are grateful to Professor Xing Xie and his research team for their help and support to realize several experiments in the Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
We like to thank also the pathology division team, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China, for their help.
Compliance with Ethical Standards
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- GLOBOCAN 2012: Estimated cancer incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Tjalma W.A, Weyler J.J, Bogers J.J, Pollefiet C, Baay M, Goovaerts G.C, Vermorken J.B, van Dam PA, van Marck E, Buytaert P.M. The importance of biological factors (bcl-2, bax, p53, PCNA, MI, HPV and angiogenesis) in invasive cervical cancer. Eur J Obst Gynecol Reprod Biol. 2001;97:223-30.
CrossRef - Walboomers J.M, Jacobs M.V, Manos M.M, Bosch F.X, Kummer J.A, Shah K.V,Snijders P.J, Peto J, Meijer C.J, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-9.
CrossRef - Filho L.A, Utagawa M.L, Shirata N.K, Pereira S.M, Namiyama G.M, Kanamura C.T, Gda S.C, de Oliveira M.A, Wakamatsu A, Nonogaki S, Roteli-Martins C, di Loreto C, de Castro M, Mda F.G, Maeda M.Y, Alves V.A, Syrjänen K. Immunocytochemical expression of p16INK4A and Ki‑67 in cytologically negative and equivocal pap smears positive for oncogenic human papillomavirus. Int J Gynecol Pathol. 2005;24:118-24.
CrossRef - Kurvinen K, Syrjänen K, Syrjänen S. p53 and bcl‑2 proteins as prognostic markers in human papillomavirus‑associated cervical lesions. J Clin Oncol. 1996;14:2120-30.
CrossRef - Adams J.M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998; 281:1322-6.
CrossRef - Tusjimoto Y, Finger L.R, Yunis J, Nowell P.C, Croce C.M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097-9.
CrossRef - Chen-Levy Z, Cleary M.L. Membrane topology of the bcl-2 protooncogenic protein demonstrated in vitro. J Biol Chem. 1990;265:L4929-33.
- Krajewski S, Tanaka S, Takkayama S, Schibler M.J, Fenton W, Reed J.C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53: 4701-14.
- Reed J.C. Bcl-2 and regulation of programmed cell death. J Cell Biol. 1994;124:1-6.
CrossRef - Young E, Zha J, Jockel J, Boise L.H, Thompson C.B, Korsmeyer S.J. Bad, a heterodimeric partner for bcl-XL and bcl-2, displaces bax and promotes cell death. Cell. 1995;80:285-91.
CrossRef - Liang X.H, Mungal S, Ayscue A, Meissner J.D, Wodnicki P, Hockenbery D, Lockett S, Herman B. Bcl-2 protooncogene expression in cervical carcinoma cell lines containing inactive p53. J Cell Biochem. 1995;57:509-21.
CrossRef - Levine A.J. p53, the cellular gatekeeper of growth and division. Cell. 1997;88:323-31.
CrossRef - Agarwal M.L, Agarwal A, Taylor R.W, Chernova O, Sharma Y, Stark R.G. A p53-dependent S-phase checkpoint helps to protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci. 1998;95:14775-80.
CrossRef - Ko L.J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054-72.
CrossRef - Zhang Q. Functional and physical interaction between p53 and BZLF1: implications for Epstein- Barr virus latency. Mol Cell Biol. 1994;14:1929-38.
CrossRef - Seol W.D, Chen Q, Smith L.M, Zarnegar R. Regulation of the c-met proto-oncogene promoter by p53. J Biol Chem. 1999;274:3565-72.
CrossRef - Ray R.B, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol.Chem. 1997;272:10983-86.
CrossRef - Budhram-Mahadeo V, Morris P.J, Smith M.D, Midgley C.A, Boxer L.M, Latchman D.S. p53 suppresses the activation of the BCL-2 promoter by the Brn-3a POU family transcription factor. J BiolChem. 1999;274:15237-44.
CrossRef - Myashita T, Krajewski S, Krajewska M, Wang H, Lin H.K, Hoffman B, Lieberman D, Reed J.C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-805.
- Greenblatt M.S, Bennel W.P, Hollstein M, Harris C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855-78.
- Nayak B.K, Baral R.N, Das B.R. p53 gene mutation in relation to p53 protein accumulation in male and female breast cancer. Neoplasma. 1996;43:305-10.
- Masuda H, Miller C, Koeffler H.P, Baflifora H, Cline M. Rearrangement of p53 gene in human osteosarcomas. Proc Natl Acad Sci. 1987;84:7716-19.
CrossRef - Bremer G.L, Tieboschb A.T.M.G, van der Putten H.W.H.M, de Haan J, Arends J.W. p53 tumor suppressor gene protein expression in cervical cancer: relationship to prognosis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1995;63:55-59.
CrossRef - Crook T, Vousden K.H. Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J. 1992;11:3935-40.
- Fujita M, Inoue M, Tanizawa O, Iwamoto S, Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. 1992;52:5323-27.
- Thomas M, Pin D, Banks L. The role of E6 and p53 interaction in molecular pathogenesis of HPV. Oncogene. 1999;18:7690-700.
CrossRef - Grace V.M, Shalini J.V, lekha T.T, Devaraj S.N, Devaraj H. Co‑overexpression of p53 and bcl‑2 proteins in HPV‑induced squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2003;91:51-8.
CrossRef - Dimitrakakis C, Kymionis G, Diakomanolis E, Papaspyrou I, Rodolakis A, Arzimanoglou I, Leandros E, Michalas S. The Possible Role of p53 and bcl-2 Expression in Cervical Carcinomas and Their Premalignant Lesions. Gynecologic Oncology. 2000;77:129-36.
CrossRef - Dako (catalogue). Monoclonal Mouse Anti-Human BCL2 Oncoprotein, Clone 124, Code M0887. 2002.
- Dako (catalogue). Monoclonal Mouse Anti-Human p53 Protein, Clone DO-7, Code M 7001. 2002.
- Tjalma W, De Cuyper E, Weyler J, van Marck E, DePooter C, Albertyn G, van Dam P. Expression of bcl-2 in invasive and in situ carcinoma of the uterine cervix. Am J Obstet Gynecol. 1998;178:113-17.
CrossRef - Cattoretti G, Rilke F, Andreola S,D, Amato L, Delia D. p53 expression in breast cancer. Int. J. Cancer. 1988;41:178-83.
CrossRef - Gronostajski R.M, Goldberg A.L, Pardee A.B. Energy requirement for degradation of tumor-associated protein p53. Mol.Cell.Biol. 1984;4:442-48.
CrossRef - Finlay C.A, Hinds P.W, Tan T.H, Eliyahu D, Oren M, Levine A.J. Activating mutations for transformation by p53 produce a gene product that forms an hsc 70-p53 with an altered half-life. Mol.Cell.Biol. 1988;8:531-39.
CrossRef - Akasofu M, Oda Y. Immunohistochemical detection of p53 in cervical epithelial lesions with or without infection of human papillomavirus types 16 and 18. Virchows Arch. A Pathol.Anat .Hist. 1995;425:593-602.
- Cheah P.L, Looi L.M. p53 immunohistochemical expression: Messages in cervical carcinogenesis.Pathology. 2002;34:326-31.
CrossRef - Tan G.C, Sharifah N.A, Salwati S, Shiran M.S, Hattaa A.Z, Ng H.O. Immunohistochemical study of p53 expression in premalignant and malignant cervical neoplasms. Med.Health. 2007;2:125-32.
- Baskaran K, Karunanithi S, Sivakamasundari I, Sundresh N.J, Thamaraiselvi B, Swaruparani S. Overexpression of p53 and its role as early biomarker in carcinoma of uterine cervix. Int .J. Res.Pharm Sci. 2013;4:198-202.
- Shukla S, Dass J, Pujani M. p53 and Bcl-2 expression in malignant and premalignant lesions of uterine cervix and their correlation with human papilloma virus 16 and 18. South Asian Journal of Cancer. 2014;3:48-53.
CrossRef - Chen H.Y, Hsu C.T, Lin W.C, Tsai H.D, Chang W.C. Prognostic value of p53 expression in stage IB1 cervical carcinoma. Gynecol Obstet Invest. 2000;49:266-71.
CrossRef - Rajkumar T, Rajan S, Baruah R.K, Majhi U, Selvaluxmi G, Vasanthan A. Prognostic significance of Bcl‑2 and p53 protein expression in stage IIB and IIIB squamous cell carcinoma of the cervix. Eur J Gynaecol Oncol. 1998;19:556-60.
- Ngan H.Y, Cheung A.N, Liu S.S, Cheng D.K, Ng T.Y, Wong L.C. Abnormal expression of pan-ras, c-myc and tp53 in squamous cell carcinoma of cervix: Correlation with HPV and prognosis. Oncol Rep. 2013;8:557-61.
- Caamano J, Ruggeri B, Momiki S, Sickler A, Zhang S.Y, Klein-Szanto A.J.P. Detection of p53 in primary lung tumors and non-small cell lung carcinoma cell lines. Am J Pathol.1991;139:839-45.
- Werness B.A, Levine A.J, Howley P.M. Association of human papillomavirus 16 and 18 E6 proteins with p53. Science. 1990;248:76-9.
CrossRef - Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci; 88:5523-7 (1991).
CrossRef - Scheffner M, Werness B.A, Huibregtse J.M, Levine A.J, Howley P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-36.
CrossRef - Lakshmi S, Nair M.B, Jayaprakash P.G, Rajalekshmy T.N, Nair M.K, Pillai M.R. p53 protein and tumorigenesis in the uterine cervix. Gen Diagn Pathol. 1997;142:281-7 .
- Mittal K.R, Lin O, Chan W, Goswami S, Demopoulos R.I. Cervical squamous dysplasias and carcinomas with immunodetectable p53 frequently contain HPV. Gynecol Oncol. 1995;58:289-94.
CrossRef - Ngan H.Y, Tsao S.W, Liu S.S, Stanley M. Abnormal expression and mutation of p53 in cervical cancer-a study at protein, RNA and DNA levels. Genitourin Med. 1997;73:54-8.
CrossRef - Munakata S, Watanabe O, Ohashi K, Morino H. Expression of Fas ligand and bcl‑2 in cervical carcinoma and their prognostic significance. Am J Clin Pathol. 2005;123:879‑85.
CrossRef - Wootipoom V, Lekhyananda N, Phungrassami T, Boonyaphiphat P, Thongsuksai P. Prognostic significance of Bax, Bcl‑2, and p53 expressions in cervical squamous cell carcinoma treated by radiotherapy. Gynecol Oncol. 2004;94:636‑42.
CrossRef - Silvestrini R, Veneroni S, Daidone M.G, Benini E, Boracchi P, Mezzetti M, Fronzo D.G, Rilke F, Veronesi U. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994;86:499-504.
CrossRef







