Manuscript accepted on :January 20, 2018
Published online on: --
Plagiarism Check: Yes
A. Nizar Ahmed1 , Ramakrishnan2 and Dj. Victor3
, Ramakrishnan2 and Dj. Victor3
1Faculty of Dental sciences Sri Ramachandran university Porur Chennai.
2Adhiparashakthi Dental College and hospital Melmaruvathur.
3Srm Dental college and hospital Ramapuram Chennai.
DOI : https://dx.doi.org/10.13005/bpj/1403
Abstract
To identify the presence of periodontal pathogens Tannerella Forsythia (T.f) and Treponema Denticola (T.d) in Down syndrome (DS) and systemically healthy subjects with periodontitis. 60 age matched subjects were categorized into three groups; Group I: 20 DS subjects with periodontitis, Group II: 20 Systemically healthy subjects with periodontitis, Group III: 20 Systemically healthy subjects without periodontitis. Plaque samples from all the three groups were collected and analyzed to evaluate the presence of T.f and T.d using Polymerase Chain Reaction. The indices that were used include Oral Hygiene Index Simplified, Community Periodontal Index For Treatment Needs and Plaque index. This study showed a statistically significant detection in the levels of T.f and T.d in DS. There was a statistically significant presence of T.d and T.f in DS subjects compared to systemically healthy periodontitis and controls.
Keywords
Chronic Periodontitis; Down Syndrome; Oral Hygiene; Subgingivalmicro Flora Taneralla Forsythia; Treponema Denticola;
Download this article as:| Copy the following to cite this article: Ahmed A. N, Ramakrishnan R, Victor D. Identification of Tannerella Forsythia and Treponema Denticola in Down Syndrome Subjects and Healthy Subjects with Periodontal Disease- A PCR Study. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Ahmed A. N, Ramakrishnan R, Victor D. Identification of Tannerella Forsythia and Treponema Denticola in Down Syndrome Subjects and Healthy Subjects with Periodontal Disease- A PCR Study. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18715 |
Introduction
Down syndrome was unheard of until the late nineteenth century. John Langdon Down, an English physician in 1866, first published an accurate description of a person with Down syndrome. This earned him recognition as the “Father of Down syndrome”. Human somatic cells contain 46 chromosomes organized into 23 pairs. Each pair has one chromosome inherited from each parent. Presence of third copy or extra copy of chromosome 21 is called as Down syndrome it is a genetic associated disorder.1
It is most commonly caused due to an error in cell division known as non dis junction. However, other chromosomal abnormalities such as mosaicism and translocation have also implicated, although to a much lesser extent. Trisomies can be classified in to four division depends on the triplicated genomic region a. complete chromosome trisomies, b. partial trisomies, c. microtrisomies and d. triplication of single functional genomic elements. DS subjects exhibits marginal gingivitis, chronic periodontitis with furcation involvement, increased tooth mobility eventually leads to tooth loss because of poor oral hygiene over a period of time.2,3 DS individuals are more prone to periodontal breakdown when compared with similar age group of normal individuals and 60 to 90 % increased disease severity in other mental disabilities.4 Bone loss was increased in Ds individuals between 35% to 74%which has been described by the Agholme et al. in 1999 in his longitudinal study. In the previous literatures disease severity and progression was exacerbated.5 To rule out the occurrence of early onset of periodontal disease in DS children is difficult because most of them had poor oral hygiene.6 simultaneously, indivivduals with DS have been shown to have moderate association between severity of periodontitis and dental plaque.3 Hence severity of periodontal breakdown cannot be elucidated by poor oral hygiene alone in DS individuals.4 Many studies have shown an increase in species like Aggregatibacter actinomycetemcomitans, Prevotella gingivalis, Prevotella intermedia, Tannerella Forsythia, Treponema Denticola, Capnocytophaga ochracea, Capnocytophaga sputigena and Eikenella Corrodens in DS subjects with periodontitis.
The current study hypothesizes that the presence of T.f and T.d, predisposes DS subjects to severe periodontal destruction. Hence, a study protocol was designed to correlate the presence of T.f and T.d in DS children with periodontal diseases which was compared to systemically healthy controls with periodontal diseases.
Materials and Methods
Study Subjects and Setting
Twenty DS subjects were considered as part of the experimental group and 20 age-matched systemically healthy individuals were included as controls in this study. DS subjects samples were taken from Balavihar School of Special Children, Kilpauk, Chennai, Tamil Nadu. Systemically healthy age-matched controls with periodontitis were taken from the patients attending the Department of Periodontology, Thai Moogambigai Dental College and Hospital, Maduravoyal, Chennai. The Institutional Ethical committee approval was obtained for the study. Due consent was obtained from the head of Balavihar School of Special Children, Kilpauk, Chennai and all control subjects recruited from Thai Moogambigai Dental College and Hospital signed an informed consent.
The DS and controls were categorized into three groups. The experimental group (Group I) included 20 DS subjects with periodontitis and the control groups, Group II had 20 systemically healthy subjects with periodontitis and Group III had 20 systemically healthy subjects without periodontitis. The grouping and presence of calculus, bleeding on probing, pocket were assessed on the basis of their Community Periodontal Index for Treatment Needs (CPITN) scores. Subjects with mental retardation, endocrinal disease, coronary heart disease, smokers, pan chewers and other systemic diseases were excluded from the study. Simplified oral hygiene indexed used to assess the oral hygiene status.
Collection of Plaque Sample
Subgingival plaque sample were collected from the deepest pocket of mesial and buccal sites of teeth with a sterile paper point (no. 35, DiaDent, Almere, The Netherlands), Samples were placed in 0.1 ml ethanol (99.9% pure, M.W. 46.08). The samples were immediately transferred in to −80°C deep freezer and processed at the Central Research Laboratory at Meenakshi Ammal Dental College and Hospital, Chennai. The samples were then analyzed for Identification of T.f and T.d using Polymerase Chain Reaction (PCR). The processing reagent, PCR reagents and Master Mix Kit were obtained from Applied Biosystems, Warrington, UK.
Primer design and selection Species-specific primers (Eurofins Genomic Pvt. Ltd. And Biosource & Surgicals ) were used to detect the presence of the T.f and T.d The expected product lengths T.f were 641 bp length. The expected product lengths T.d were 311 bp.q A pair of ubiquitous primers product length (602 bp), (which matched most bacterial 16S rRNA genes at the same position) was used as a positive control for the PCR reaction.[Table 1]
Processing of Samples
(mRNA isolation) Collected plaque samples were stored in Eppendorf tubes containing PBS solution at −80°C.Genomic DNA was extracted using a QIAamp DNA Mini kit (QIAGEN Inc., USA, 9300 Germantown Road, Germantown, MD 20874). All steps were carried out at room temperature. (According to manufacturer’s instructions)Subgingival plaque samples containing bacteria cells were centrifuged for 10 min at 7500 rpm to become pellet. Pellet of bacteria was put in 180 μl of the solution containing (2 mM EDTA; 1.2% Triton 20 mg/ml lysozyme ; pH 8.0, 20 mM,Tris-HCl ). Later, incubated for 30 min at 37°C . 20μl proteinase K and 200μl Buffer AL. was mixed by means of vortex. Incubating samples at 56°C for 30 min and then at 95°C further incubation should be done. After incubation, the samples were centrifuged for a few seconds. Ethanol 200μl (96–100%) was incorporated with the sample, and vortexed for 15 s. The contents were transfer to QIAamp Mini spin column (in a 2 ml collection tube) and centrifuged at 8000 rpm for 1 min and eluted with 100μl buffer. The purity of DNA sample was checked by the nano drop method using multi-wavelength program of (260/280 nm).
Quantitative RT-PCR was performed with the Stratagene MX3000P (Agilent technologies, 5301 Stevens Creek Blvd. Santa Clara, CA 95051). The double standard DNA-binding dye SYBR Green I (KAPA SYBR FAST qPCR Kit) using species-specific primers used for Tannerella forsythus and Treponema denticola [Table 1].
Table 1: PCR primer sequence Target Polymerase chain reaction primer pairs.
Tannerella Forsythus
Forward-GCG TAT GTA ACC TGC CCG CA
Reverse- TGC TTC AGT GTC AGT TAT ACC T
Treponema Denticola
Forward- AAG GCG GTA GAG CCG CTC A
Reverse- AGC CGC TGT CGA AAA GCC CA
The reactions were performed as triplicates. No template control (NTC) were used. Melt curve analysis was performed using the thermal cycling programmed at 59°C-95°C . 2% agarose gel electrophoresis was performed to resolve the PCR products (control and test groups) along with 100bp ladder. Melting curves generated in the Real time PCR was compared to products observed in the agarose gel for the presence of presence of a single PCR product and appropriate sized amplicon.
Statistical Analysis
Each group mean and standard deviation was calculated . Comparing the mean value among different study groups by using one-way ANOVA Kruskal-Wallis followed by post-hoc (Tukey HSD) test. The statistical analysis was performed using SPSS software version 17.0.0 (SPSS manufacturer IBM Corporation, 1 New Orchard Road, Armonk, New York 10504-1722, United States. P < 0.05 is considered level of significance.
ANOVA for T. Denticola
The Ct values were compared for T. Denticola between groups and within groups which reveales a high statistical significance in the level of T. Denticola in DS subjects when compare to systemically health chronic periodontitis and systemically healthy controls with the p value of 0.000(p<0.05) [Table 2].
Table 2: shows Oneway Anova for T. Denticola Levels
| Groups | N | Mean | Std. Deviation | Sig. |
| Down syndrome with Ch. Periodontitis | 20 | 18.6155 | .27190 | .000 |
| Systemically healthy with Ch. Periodontitis | 20 | 24.9555 | .81561 | |
| Control | 20 | 34.8816 | .56946 | |
| Total | 60 | 26.1509 | 6.77600 |
In this study T.d levels were elevated in DS subjects with periodontitis compared with the systemically healthy with chronic periodontitis and systemically healthy controls. However the levels of T.d in DS periodontitis group were most significant when compared to T. Forsythia.
The post‑hoc test for multiple comparisons of T.d among the three groups showed a highly statistical significance in the levels of T.d in DS periodontitis group when compared to control groups with P= 0.000 [Table 3].
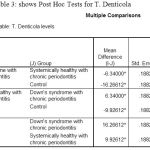 |
Table 3: shows Post Hoc Tests for T. Denticola
|
ANOVA for Tannerella forsythus
The comparison of Ct values of B. Forsythus within groups by oneway ANOVA revealed a statistically highly significance in the level of T.f in Ds with chronic periodontitis subjects when compare to systemically health chronic periodontitis and systemically healthy controls with the p value of 0.000(p<0.05) [Table 4].
Table 4: shows Oneway Anova for T. Forsythus
| Groups | N | Mean | Std. Deviation | Sig. |
| Down syndrome with Ch. Periodontitis | 20 | 18.9895 | .49228 | .000 |
| Systemically healthy with Ch. Periodontitis | 20 | 23.1875 | .73128 | |
| Control | 20 | 35.9359 | .95915 | |
| Total | 60 | 26.0376 | 7.30423 |
The post‑hoc test for multiple comparisons of T.forsythus among the three groups showed a high statistical significance in the levels of T.f in DS periodontitis group when compared to Ds with systemically healthy chronic periodontitis and Systemically healthy control groups with P = 0.000 respectively, similarly the test revealed a statistically significant level of T.d is in comparison with T.f in DS periodontitis group [Table 5].
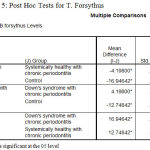 |
Table 5: Post Hoc Tests for T. Forsythus
|
Discussion
DS subjects have more susceptible to early loss of teeth due to poor oral hygiene.7 DS subjects exhibits the chaos of immune response like altered polymorphonuclear neutrophil, monocyte and reduction in count of cluster of differentiation 4 cells and T-lymphocytes is a reason behind periodontal disease progression. However, the basis of these immune defects is unclear. A plenty of factors have been postulated in the induction of immunodeficiency, such as zinc deficiency and accelerated immunosenescence, although the clinical significance is yet to be established. The periodontal status among DS individuals has seen a clear improvement in the recent years owing to an increased awareness and prompt dental care. In 35% of DS adolescence exhibited early signs of alveolar bone loss pertaining to the lower anteriors and oral health of DS was alike of Early periodontitis.8 The Periodontal disease severity cannot determined by the sole factor of plaque and calculus. Thus, an abnormal capillary morphology, connective tissue disorders and anatomical aspects of teeth might contribute to the periodontal pathology.9
In this study, DS with periodontitis subjects showed a statistically significant increase in the levels of both T.d (P < 0.00) and T.f (P < 0.000) when compared with systemically healthy periodontitis and controls; however both organisms were found to be increased in DS periodontitis group. The results of this study are in accordance with Amano et al.10 who stated that an increase in T.d and T.f levels in DS gingivitis subjects played an key role in the onset of gingival inflammation followed by the plaque maturation.
Increased levels of T.d (P < 0.000) and T.f (P < 0.000) were seen in DS group when compared to systemically healthy with periodontitis group and healthy controls. The results were similar to the study done by Amano et al. and Agholme et al. The reason for prevalence of these organisms in DS patients could be attributed to an early colonization of these organisms since childhood.10,11 Multiple comparisons within the three groups using post‑hoc test showed that the Ct levels of T.d and T. f were highly statistically significant in DS periodontitis group. The results of this study correlated with the study done by Sakellari et al.12 However Amano et al.13 and Amano et al.[10]in their studies on DS subjects without controls had shown elevated levels of T.d and T.f respectively.
This is one of the first studies to evaluate and quantify specific periodontal pathogens in DS subjects amongst the Indian subpopulation and compare the same with healthy controls. Within the limits of the study, it can be concluded that the presence of T.d and T.f with a probable compromised immune response exist in DS subjects, predisposing them to gingival inflammation and possible early onset periodontitis. Further studies are needed to evaluate multiple periodontal pathogens and simultaneously assess the immune cell functions that could contribute to the disease process thereby broadening our understanding on the etiopathogenesis of diseases of periodontium in DS individual.
Conclusion
The Current study revealed a highly significant presence of potent periodontal pathogens T.d and T.f in DS subjects compared to systemically healthy periodontitis and controls.
Conflict of Interest
There is no conflict of interest
Funding Source
Self funding
References
- Langlais R.P, Miller C.S, Nield-Gehrig J.S. Color Atlas of Common Oral Diseases. 4th ed. Philadelphia: Lippincott Williams and Wilkins. 2009.
- Shaw L, Saxby M.S. Periodontal destruction in Down’s syndrome and in juvenile periodontitis. How close a similarity? J Periodontol. 1986;57:709-15.
CrossRef - Langlais R.P, Miller C.S, Nield-Gehrig J.S. Color Atlas of Common Oral Diseases. 4th ed. Philadelphia: Lippincott Williams and Wilkins. 2009.
- Cutress T.W. Periodontal disease and oral hygiene in trisomy 21. Arch.Oral. Biol. 1971;16:1345-55.
CrossRef - Agholme M.B, Dahllöf G, Modéer T. Changes of periodontal status in patients with Down syndrome during a 7-year period. Eur .J. Oral Sci. 1999;107:82-8.
CrossRef - Bradley C, McAlister T. The oral health of children with Down syndrome in Ireland. Spec Care Dentist. 2004;24:55-60.
CrossRef - Shaw L & Saxby M.S. Periodontal destruction in Down syndrome and in juvenile periodontitis: how close a similarity? J Periodontol. 1986:57(11):709-713.
CrossRef - Ram G and Chinen J. Infections and immunodeficiency in Down syndrome. 2011;164:9–16.
- Reuland‑Bosma W, Dijk v.J. Periodontal disease in Down syndrome: A review. J Clin Periodontol. 1986;13:64‑73.
CrossRef - Amano A, Kishima T, Akiyama S, Nakagawa I, Hamada S, Morisaki I. Relationship of periodontopathic bacteria with early‑onset periodontitis in Down’s syndrome. J Periodontol. 2001;72:368‑73.
- CrossRef
- Agholme M.B, Dahllöf G, Modéer T. Changes of periodontal statusin patients with Down syndrome during a 7‑year period. Eur .J. Oral Sci. 1999;107:82‑8.
- Sakellari D, Arapostathis K.N, Konstantinidis A. Periodontal conditions and subgingival microflora in Down syndrome patients. A case‑control study. J Clin Periodontol. 2005;32:684‑90.
CrossRef - Amano A, Kishima T, Kimura S, Takiguchi M, Ooshima T, Hamada S, et al. Periodontopathic bacteria in children with Down syndrome. J Periodontol. 2000;71:249‑55.
CrossRef







