Manuscript accepted on :October 16, 2017
Published online on: --
Sujatha P , Preethi G. Anantharaju
, Preethi G. Anantharaju , Prashanthkumar M. Veeresh
, Prashanthkumar M. Veeresh , Sumit Dey
, Sumit Dey , Venugopal Reddy Bovilla
, Venugopal Reddy Bovilla and Subba Rao V. Madhunapantula
and Subba Rao V. Madhunapantula
Center of Excellence in Molecular Biology and Regenerative Medicine (CEMR), Department of Biochemistry, JSS Medical College, Jagadguru Sri Shivarathreeshwara University, Mysuru - 570 015, Karnataka, India.
Corresponding Author E-mail: mvsstsubbarao@jssuni.edu.in
DOI : https://dx.doi.org/10.13005/bpj/1273
Abstract
Diallyl disulfide (DADS) is sulfur containing anti-cancer agent derived from garlic. Several in vitro studies have demonstrated the ability of DADS to inhibit the growth of cancer cells. However, only few studies have tested the ability of DADS to retard the development of tumor cells in animal models. Therefore, in this study we have measured the anti-tumor efficacy of DADS using well-established EAC tumor model representing a liquid-tumor. Analysis of the data showed a significant decrease in tumor growth as evidenced by a significant reduction in animal body weight in liquid tumor model. Mechanistically, DADS induced apoptosis by promoting caspase-3 expression and preventing the oxidative degradation of anti-tumor proteins such as p53 by upregulating antioxidant enzymes NQO1 and SOD; and reducing agents such as GSH. In summary, DADS appears to be a potential anti-cancer agent for considering it as a monotherapy or for selecting it as a component drug in the combination trials.
Keywords
Breast Cancer; Diallyl Disulfide; EAC Model; Garlic Tumor Growth;
Download this article as:| Copy the following to cite this article: Sujatha P, Anantharaju P. G, Veeresh P. M, Dey S, Bovilla V. R, Madhunapantula S. R. V. Diallyl Disulfide (DADS) Retards the Growth of Breast Cancer Cells in Vitro and in Vivo Through Apoptosis Induction. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Sujatha P, Anantharaju P. G, Veeresh P. M, Dey S, Bovilla V. R, Madhunapantula S. R. V. Diallyl Disulfide (DADS) Retards the Growth of Breast Cancer Cells in Vitro and in Vivo Through Apoptosis Induction. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17557 |
Introduction
Diallyl disulfide (DADS) is an organosulfur compound derived from garlic (Allium sativum) with proven health beneficial effects.1 Various cell-based assays determining the anticancer activity of DADS have shown the ability of this compound to inhibit proliferation of cell lines representing carcinomas of breast, prostate, lung and liver.1,2, 3 Mechanistic studies delineating the pathways involved in cancer cell growth inhibition have demonstrated (a) upregulation of apoptotic signaling, especially caspase-3 and cleaved PARP; (b) induction of cell cycle arrest at G0/G1 and G2/M phases; (c) inhibition of proliferation cascades (d) downregulation of cell migration and (e) activation of anti-oxidant response genes including Nrf2 and its target proteins NQO1 etc.1,4,5 Likewise, recent studies have identified induction of p53 and p21 in esophageal squamous cell carcinomas.5
In animals, DADS was shown to retard the growth of subcutaneous xenografted tumors.6 Few studies have also demonstrated the ability of DADS to retard EAC cell growth in the peritoneal cavity of mice. However, the molecular mechanisms responsible for DADS-induced EAC cell death are not known. A separate study has demonstrated the ability of Garlic Oil (rich in DADS) to halt the development of EAC solid tumors.7 This study has shown inhibition of HDAC and induction of antioxidant catalase (CAT), glutathione-S-transferase (GST) and glutathione (GSH) levels. But, not much is known about which master regulator is responsible for these molecular changes occurred due to Garlic Oil administration.7 Further it is also not studied whether these molecular changes are due to DADS or any other sulfur containing compound present in garlic oil.8
Therefore, in this study we have determined the efficacy of DADS to inhibit triple negative breast cancer cell line MDA-MB-468 and compared the potential with normal lung epithelial cell line BEAS-2B. Next, the ability of DADS to inhibit intraperitoneally growing EAC cells was assessed in mice. Effect of DADS on the expression of proteins such as NQO1, SOD and Caspase, which are involved in antioxidant activity and apoptosis induction were also measured. In summary, we could demonstrate that DADS inhibit breast cancer cell growth in vitro as well as in vivo by promoting apoptosis as well as by triggering antioxidant proteins, which subsequently stabilize the oxidative degradation of tumor suppressor proteins such as p53. Stabilized p53 promotes apoptosis in cells leading to tumor growth inhibition.
Materials and Methods
Materials
85% pure (by HPLC) diallyl disulfide (IUPAC Name: 4, 5-dithia-1, 7-octadiene; Mol. Wt: 146.28g/mol) was procured in oil form from Alfa Aesar Haverhill, Massachusetts USA. Cancer cell line MDA-MB-468 (ER, PR and HER2 negative) was procured from National Center for Cell Science, Pune, Maharashtra, India. Cell culture consumables including DMEM with phenol red, with 4.5g/L glucose, but lacking L-glutamine and sodium bicarbonate, fetal bovine serum, trypsin, glutamax, phosphate buffered saline, pen-strep antibiotic mixture were procured from Life Technologies, USA. All other reagents were of analytical grade. Swiss Albino mice were procured from central animal facility, JSS University, Mysuru.
Methods
Purity of DADS
Purity of DADS was assessed by injecting 10µL of DADS from 0.01mg/mL to 10.0 mg/mL on Shim-Pack VP-ODS (C18 column, 250mm x 4.5mm), and by eluting the loaded sample using Acetonitrile : Water (75:25) as mobile phase at a flow rate of 1mL/min. The eluted DADS was detected at 240nm.
Determination of anti-cancer Activity of DADS
The anti-cancer activity of DADS was measured according to Madhunapantula et al., 2008 (9). In brief 0.5 X 104 MDA-MB-468 cells (representing carcinomas of breast) in 100µL DMEM supplemented with 10% FBS were seeded in a 96-well plate and incubated at 37oC in a cell culture incubator maintained at 5% CO2 and 95% relative humidity. After 48h, the cells were exposed to increasing concentration (62.5µM to 1000µM) of DADS (dissolved in DMSO and diluted in DMEM-10% FBS medium) for 24 and 48h and the viability of cells measured using sulforhodamine-B and MTT assays. Camptothecin (40µM) was used as positive control for drug treatment
Measurement of Cell Viability using Sulforhodamine-B assay (SRB assay)
SRB assay was performed according to Skehan et al., 1990.10 Experimentally, cells were fixed in 1/4th volume of cold 50% (w/v) TCA for 4˚C. After 1h, the media was removed and the wells washed with water (200µL X 4 times) to remove TCA and serum proteins. The plates were dried, incubated with 100µL 0.4% SRB (in 1% acetic acid) for 30.0 minutes to stain the cellular proteins. Unbound SRB was removed by washing quickly with 1% acetic acid (200µL X 4 times). The bound SRB was solubilized in 10.0mM Tris base solution (100µL/well) and the absorbance measured in a multimode plate reader operating at 490nm. Percentage cell viability was calculated using the following equation
% Viability = 100- {(OD of Control – OD of Sample) / OD of Control) x 100}
Measurement of Cell Viability using MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay
First, a stock of 12mM MTT was prepared by adding 1mL of sterile PBS to 5mg of MTT. For measuring the viability 20µL 12mM MTT was added to cells (untreated and treated) growing complete medium. The plate was incubated for 1h 10minutes in CO2 incubator at 37oC added centrifuged at 3500rpm for 5miniutes. The supernatant was discarded and the pellet was dissolved in 100µL of DMSO and read at 570nm in a multimode plate reader.
Detection of Apoptosis by Acridine Orange and Ethidium Bromide Staining
Apoptosis detection using acridine orange and ethidium bromide staining method was carried out as described by Shailasree et al., 2015.11 In brief, first, 0.3 x 106 MDA-MB-468 cells were plated in 6-well plates and allowed to grow for ~36h. The exponentially growing cells were exposed to increasing concentrations of DADS (500µM, 1000µM and 1500µM) for about 48h. The control and treated cells were trypsinized and mixed thoroughly to obtain a single cell suspension. Trypsin was neutralized by the addition of complete medium. An about 20.0μL cell suspension was incubated with 10.0μL ethidium bromide (100.0µg/mL) and 10.0μL of acridine orange (100.0µg/mL) mixture for 10.0 minutes. The cells were imaged using the fluorescence microscope using TRITC and FITC filters. The images obtained using 2 different channels were merged to obtain a combined image, which emitted green and orange cells.11 The live cells take up acridine orange (which stains the cells green), while the apoptotic cells, (whose membrane integrity is lost and nucleus is exposed) take up the ethidium bromide and appear orange when photomicrographed under fluorescence microscope.
Evaluation of the Efficacy of Diallyl Disulfide for Retarding EAC Cells Growing in the Peritoneal Cavity of Mice
The study was carried out after receiving the approval from the Institutional Animal Ethics Committee, JSS College of Pharmacy (208/2016), Mysuru. Experimentally, first, 6-8 weeks old female Swiss albino mice (n = 18) weighing around 25-28g were injected with 1 x 106 viable EAC cells in to the peritoneal cavity. The EAC cells were collected from ascites fluid of a mouse already harboring healthy EAC cells. The collected EAC cells were diluted with PBS, and number of viable cells quantitated using Tryphan blue exclusion method.12 After 24h of tumor cells injection, the mice were divided in to 3 groups (n=6/group). Animals in Group-II (injected with EAC cells) were administered with vehicle 50% DMSO in PBS (50µL/mouse, intra peritoneal), the animals in Group-III received DADS 50mg/kg body weight (50µL/mouse intra peritoneal). A set of 6 animals not injected with EAC cells (Group-I) were used as controls for measuring the normal body weight gain (No tumor). These animals were, however, injected with 50% DMSO in PBS (50µL/mouse) to measure whether DMSO vehicle alone has any influence on body weight gain. The treatment was continued for 18 days by injecting the drugs on alternative days. Body weight was recorded every other day. On 19th day mice were sacrificed by cervical dislocation and the ascites collected were used for estimation of SOD, GSH, and Caspase-3 activity. The vital organs were collected and the H and E staining was carried out.13
Colorimetric Estimation of Caspase-3 Activity using A Chromogen-Coupled DEVD Substrate
The caspase-3/CPP32 colorimetric assay kit was used to measure the caspase-3 activity [http://www.biovision.com/manuals/K106.pdf] in EAC cells collected from tumor kinetics experiment. Protein lysates from EAC cells collected from control and DADS treated animals were prepared using 100μL of lysis buffer provided in the kit. Total protein content in the cell lysates was estimated using BCA method, and caspase-3 activity determined by incubating 100.0μg of total protein in a total volume of 50.0μL cell lysis buffer with 5.0μL 4.0mM DEVD-pNA substrate (200.0μm final concentration) and 50μL of 2X reaction buffer containing 10.0mM DTT at 37˚C for 3h. The developed color was read at 405nm using a multimode plate reader (PerkinElmer). The fold change compared to control untreated cells was calculated and plotted against compound concentration.
Evaluation of Antioxidant Enzyme SOD and Reduced Glutathione (GSH) Levels
The levels of antioxidant enzyme SOD and the intracellular metabolite – reduced glutathione (GSH) was measured in EAC cells collected from mice treated with experimental DADS and control 50% DMSO vehicle, according to method described by Sharma et al 2009 and Sun Y et al 1988.14,15 Experimentally, EAC cells were washed thrice with PBS to remove the traces of peritoneal fluid. To the pellet 100-150 µL of ice-cold RIPA buffer was added and incubated for 30 minutes on ice. The lysed cells were centrifuged at 14000 rpm for 30 minutes and the supernatant collected. Total protein in the collected protein lysates was estimated using the BCA method.
Measurement of SOD: Superoxide dismutase enzyme catalyzes the conversion of super oxide anions into less destructive molecules thus making them an important antioxidant defense system.16 The levels of SOD in EAC cells collected from mice treated with experimental DADS and control 50% DMSO vehicle was measured by the method detailed by Sun Y et al 1988.15 First, cell lysates containing 2,4,6,8 and 10 µg of total protein in a final volume of 10.0µL were incubated with 250µL of 12mM methionine. Next, 30.0µL of 0.5mM riboflavin and 10µL of 3.4mM NBT, were mixed and exposed to illumination for 5 minutes in an illumination chamber lined with aluminium foil, and fitted with a 15W fluorescent lamp. Immediately after illumination, the optical density of reaction mixture was read at 560nm. SOD substrate alone, exposed to illumination, was used as standard while a substrate without NBT served as blank. A control without NBT for each sample was also maintained and the control values were subtracted from the test values. Decrease in OD was calculated using the formula
Decrease in OD=(S-B)-(T-C)
S= Standard, B= Blank, T=Test, C=Control
SOD was expressed as units/mg protein using the formula
Formula
Measurement of reduced glutathione (GSH): Glutathione is a tripeptide antioxidant in the biological system.17 The sulphydryl group of the GSH reacts with 2-nitro benzoic acid to form a stable yellow color, which was measured at 412nm.14 100µg of the cell lysate, collected from EAC cells harvested as described above, was incubated with 100µL of the disodium hydrogen phosphate (0.3M) and 0.04% Dithiobis nitro benzoic acid (DNTB) (prepared in 0.3M disodium hydrogen phosphate) and read immediately at 412nm using the multimode plate reader. The concentration of glutathione in the samples was calculated using the calibration graph constructed using GSH (0-160µM) and expressed in µM14
Statistical Analysis
All experiments were conducted at least with 3 replicates (intra experimental) and the results were expressed as mean of 3 independent experiments + SEM calculated using GraphPad Prism version 6.0. The results were subjected to One-Way or Two-Way ANOVA to analyze differences between controls / positive controls and DADS treated samples. Tukey’s post hoc test was used after ANOVA and the “p” value of < 0.05 was considered significant.
Results
Diallyldisulfide Utilized in This Study is Pure
Diallyldisulfide (DADS, IUPAC Name: 4, 5-dithia-1, 7-octadiene; Mol. Wt: 146.28g/mol) was procured in the form of an oil with a density of 1.008. Purity of DADS was assessed by injecting 10µL of DADS from 0.01mg/mL to 10.0 mg/mL on Shim-Pack VP-ODS (C18 column, 250mm x 4.5mm), and by eluting the loaded sample using Acetonitrile : Water (75:25) as mobile phase at a flow rate of 1mL/min. The eluted DADS was detected at 240nm and the peak area was plotted against concentration to determine the linearity of standard graph (Figure 1A and 1B). A single symmetrical peak with a retention of 6.11minutes and a purity percentage of 85% was observed in the chromatogram. Two minor peaks 5.269 and 7.669 were also observed with an about 5.01% and 8.89% contribution of total area, respectively (Figure 1A). R2 value of prepared standard graph was 0.998 indicating good linearity in the concentration ranges tested (Figure 1B)
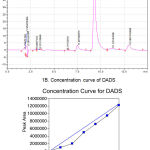 |
Figure 1: Analysis of DADS using HPLC.
|
(A) Representative chromatogram showing the elution profile of DADS. DADS was eluted as a predominant symmetrical peak at 8.2min. (B) The concentration curve of DADS. Increasing concentration of (1.0mM to 10mM) DADS was injected to C18 column and the eluted peak area plotted against concentration of DADS. A linear curve with an R2 of 0.95 was obtained.
DADS Inhibited the Growth of MDA-MB-468 Breast Cancer Cell Line More Effectively Compared to Normal Lung Epithelial Cell Line in Vitro
To determine the anti-cancer potential of DADS against a triple negative breast cancer cell line MDA-MB-468, 1.0X104 cells were plated in 100µL DMEM supplemented with 10% FBS medium and allowed to grow for about 36h in a humidified carbon dioxide incubator. Exponentially growing cells were treated with increasing concentration of DADS (46.85µM to 1500.0µM) for 24h and 48h and the viability compared with vehicle DMSO (1% final concentration) exposed cells (Figure 2A). Vehicle DMSO had only minimal effect on cell viability (<10% cell death) compared to untreated cells (Figure 2A). A dose dependent decrease in cell viability was observed with increasing concentration of DADS. At 1500.0µM concentration an about 37% cell death was observed at 24h treatment (Figure 2A). Increasing the treatment time to 48h enhanced the efficacy of DADS as an about 51% decrease in the number of viable cells was observed (Figure 2B).
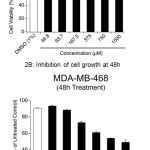 |
Figure 2: DADS inhibit breast cancer cell growth in vitro
|
(A) Inhibition of cell growth at 24h: A dose dependent reduction in cell viability was observed when, MDA-MB-468 breast cancer cells were treated with increasing concentration of DADS (from 46.8 to 1500µM) for 24h. (B) When cells were further exposed to MDA-MB-468 for 48h the cellular viability further decreased with increase in dose as determined by SRB assay.
Since DADS inhibited the triple negative breast cancer cell line MDA-MB-468, next, the efficacy on normal lung epithelial cell line BEAS-2B was tested to determine the selectivity. First, 1.0 X 104 BEAS-2B cells were plated/well in 100µL culturing medium and allowed to grow for 48h in a carbon dioxide incubator. Next, the cells were treated with increasing concentration of DADS (375.0µM, 750.0µM, 1500.0µM and 2000.0µM) for 24h and viability determined using MTT reagent as detailed in materials and methods. Analysis of the data showed a marginal cell death of 22.5% even at 1500.0µM compared to control untreated cells. DMSO (1%) alone also had an about 13.1% cell growth inhibition (Figure 3). Further increase in the concentration of DADS to 2000.0µM reduced the number of viable cells to 20% (ie., 80% inhibition) (Figure 3).
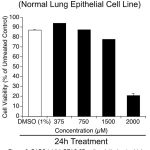 |
Figure 3: DADS inhibit BEAS-2B cells relatively at a higher dose compared to MDA-MB-468.
|
To assess whether DADS is selective to cancer cells or does it kill normal cells as well, human normal lung epithelial cells BEAS-2B were treated with increasing concentration of DADS (350.0µM, 750.0µM, 1500.0µM and 2000.0µM) for 24h and the viability measured. A significant decrease in number of viable cells was observed only at 2000.0µM DADS concentration. Not much cell death was noticed at other concentrations tested indicating that DADS has selectivity toward cancer cells
Comparison of the cell growth inhibitory potential of DADS between normal BEAS-2B cell (Figure 3) line with MDA-MB-468 cancer cell line showed more selectivity of this sulfur-containing compound to cancer cells. For example, at 24h of treatment, 1500.0µM DADS inhibited the growth of MDA-MB-468 cells by 37% (DMSO alone had no effect on cell growth), compared to normal BEAS-2B cells, where the cell growth inhibition was only 22.5% (DMSO alone had ~13% effect, resulting in an overall effect of 9.5%).
DADS-Induced MDA-MB-468 Cell Growth Inhibition Was Mediated by NQO1 Activation and Apoptosis Induction
To evaluate the mechanism of DADS induced cell growth inhibition, the MDA-MB-468 cells were exposed to acridine orange and ethidium bromide and imaged using fluorescence microscope. Live cells with intact membrane take up only acridine orange and emit green light while the dead cell where membrane integrity is lost are stained by ethidium bromide which appear red indicating apoptosis (Figure 4A) Analysis of the data showed that DADS induced dose dependent apoptosis as evident by increased orange cells (73% at 1250μM) compared to 1% DMSO treated cells (4%). In addition the DADS also elevated NQO1 activity at 250, 750 and 1250μM compared to the DMSO treatment. The increase in activity was dose dependent with 1250μM showing highest activity of 76.1% compared to DMSO which showed 52% activity (Figure 4B) Elevated NQO1 stabilizes the P53 and thereby induce apoptosis.
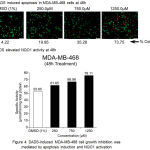 |
Figure 4: DADS-induced MDA-MB-468 cell growth inhibition was mediated by NQO1 activation and apoptosis induction.
|
(A) DADS treatment increased the NQO1 activity in MDA-MB-468 cell line. A dose dependent increase in NQO1 activity upon treatment with increasing concentration of DADS was observed in MDA-MB-468 cells indicating that NQO1 might be having a role in cell death induction. (B) Treatment of MDA-MB-468 cells with DADS elevated the levels of Caspase-3. To check whether DADS treatment induced apoptosis in breast cancer cell line, the cells were exposed to increasing concentration of DADS for 48h and the treated cells stained with Acridine Orange (AO) and Ethidium Bromide (EtBr). Analysis of stained cells using Fluorescence Microscope showed both green (Live Cells) and Red (Dead Cells) cells. A dose dependent increase in dead cell population was observed.
Intra Peritoneal Administration of DADS Retard the Growth of EAC Cells in Mice
Based on the in vitro efficacy data demonstrating the ability of DADS to inhibit breast cancer cells growth, an in vivo study using EAC tumor model was carried out to determine whether intra peritoneal administration of DADS could also retard the proliferation of cancer cells in animals. EAC is a good representative of breast cancer model in mice.18 EAC cells are undifferentiated; lack tumor-specific transplantable antigen (TSTA) and respond to drug treatment.18 Therefore, EAC cells are considered to resemble human cancer cell lines.18 Hence, in this study we have determined the efficacy of DADS for inhibiting EAC cells proliferation in vivo using female Swiss albino mice. Experimentally, first, mice were pretreated with DADS 50mg/kg body weight (i.p.) for 2 alternative days prior to induction of tumor. Next, on 3rd day 1.0 x 106 EAC cells were injected to all animals and treatment with DADS (for a group of 6 animals bearing EAC cells) was continued till 19th day by intraperitoneal injection on every alternative days. Average weight gain compared to untreated control was recorded (Figure 5). Administration of DADS showed an about 33% decrease in body weight gain of animals, compared to vehicle treated mice (Figure 5).
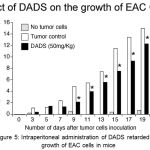 |
Figure 5: Intraperitoneal administration of DADS retarded the growth of EAC cells in mice.
|
To check whether intraperitoneal administration of DADS (50mg/kg body weight) retards the growth of EAC cells growing in the peritoneal cavity of mice, DADS was administered every other day and animal body weight measured. Whereas the control mice that received the vehicle 50% DMSO showed a significant body weight gain (an indicator of tumor cell growth) the mice that received 50mg/kg body weight DADS showed an about 30% reduction in body weight gain, indicating that DADS has anti-tumor activity.
DADS-Mediated Tumor Growth Inhibition Was Due to the Induction of Apoptosis and NQO1 Activity in Mice
Since intraperitoneal administration of DADS retarded the growth of EAC cells in mice, the mechanism(s) responsible for this effect was studied next. Experimentally, EAC cells were collected at the end of EAC tumor kinetics experiment, and protein lysates harvested by centrifugation. Caspase-3, NQO1, activity and GSH levels were measured as detailed in the materials and methods and the data compared with vehicle DMSO treated mice. A 4.49 fold increase in caspase-3 levels were observed in DADS treated mice compared to vehicle treated mice indicating that DADS induced caspase-3 mediated apoptosis (Figure 6A). In addition DADS significantly increased NQO1 activity in 50mg/kg body weight DADS treated animals (from 17.55µmoles/min/mg total protein to 26.35µmoles/min/mg total protein) (Figure 6B). Even the cellular glutathione (GSH) level, although not significant statistically, was increased upon treatment of mice with 50mg/kg DADS (Figure 6C). Corroborating with these results, a significant 3.36 fold increase in superoxide dismutase levels (from 41.19 Units in control animals to 138.29 Units) was observed in the EAC cell lysate collected from DADS treated animals (Figure 6D).
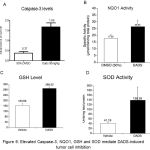 |
Figure 6: Elevated NQO1, SOD and Caspase-3 enzymes, and GSH mediate DADS-induced tumor cell inhibition.
|
To delineate the mechanisms leading to EAC cell growth inhibition, the ascites fluid from control DMSO treated and experimental DADS administered mice were collected and cell lysates prepared as detailed in materials and methods. Equal protein (from control and experimental groups) was analyzed for measuring (A) caspase-3; (B) NQO1; (C) GSH; and (D) SOD. A significant increase in NQO1, caspase-3 and SOD level was observed with DADS treatment. However, GSH showed a non-significant increase upon treatment with DADS. This data indicate that the cell death induced by DADS is in part mediated by the induction of key oxidative stress enzymes and reduced metabolite GSH
Discussion
DADS is an organo-sulfur compound derived from aged garlic.1 Many studies have demonstrated the anti-cancer potential of DADS and pointed that the cell death induced by DADS is mediated through the activation of apoptosis, cell cycle arrest and inhibition of proliferation and angiogenesis.19 However, these effects are dose dependent and the treatment outcomes and the mechanisms being operated in cancer cells vary with type of tumor being treated as well as the stage at which it is exposed to DADS.19 For example, consumption of aged garlic (rich in DADS) prevents the transformation of normal cells to cancerous ones by protecting the cells from oxidative stress through the induction antioxidant enzymes NQO1, SOD and GST.1 Supporting this observation, many studies have shown the induction of transcription factor Nrf2 upon treatment of cells with DADS.4 Hence, DADS is considered as a good cancer preventive agent.
Recent studies have demonstrated the ability of DADS to treat advanced- and drug resistant cancer types.20 Anticancer ability of DADS is not only due to its ability to activate Nrf2 target genes such as NQO1 but also because of the potential of DADS to trigger apoptotic cell death and cell cycle arrest through the upregulation of proteins such as p53.21 Induction of NQO1 in tumor cells promotes the conversion of inactive anticancer agents in to active molecules thereby increase cell death.22 For example, a study by Pink, J.J. et al., 2000 has demonstrated that NQO1 is the key determinant of b-lapachone activity in breast cancers.23 Cells expressing NQO1 are more sensitive to b-lapachone treatment compared to NQO1 null or silenced cells.23 Therefore activation of NQO1 in cells lacking this protein sensitizes them to chemotherapeutic agents. However, it is not known how induction of NQO1 and other antioxidant proteins and metabolites is promoting cancer cell death as observed in this study. One possible explanation is that elevated expression of these proteins in DADS treated cells is a defensive mechanism of cells to avoid cell death induced by DADS through the induction of reactive oxygen species.24 Treatment of cancer cells with DADS elevates ROS levels in cells.24 A significant dose dependent increase in ROS level was observed upon treatment of breast cancer cells MDA-MB-231 and MCF-7 with DADS (data not shown). Elevated cellular ROS promotes apoptosis in cells by damaging key constituents such as DNA, proteins and lipids.24 A significant increase in the cellular apoptosis was observed when MDA-MB-468 cells were treated with DADS . Induction of apoptosis is not only due to elevated caspase-3 as observed in EAC-bearing mice treated with DADS but also could be due to enhanced p53 expression and stabilization. Elevated NQO1 is known to stabilize p53 protein in cells.25 Therefore, breast cancer cells treated with DADS are undergoing death due to induction of apoptosis, which is mediated by enhanced expression of caspase-3 proteins as well as stabilization of p53 through upregulated NQO1.
Conclusions
DADS is a potent anti-breast cancer agent, which works by promoting apoptosis through caspase-3 induction and possibly through the p53 expression. However, further studies are warranted to determine the association between NQO1 expression with p53 stabilization.
Acknowledgements
Authors would like to acknowledge DST-FIST for supporting the Department of Biochemistry, JSS Medical College, which helped us to procure key equipment utilized in this study.
Conflict of Interest
Authors have no conflict of interest
Funding Source
The Work was not supported by any Funding agency
References
- Omar S.H, Al-Wabel N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2010;18(1):51-8.
CrossRef - Butt M.S, Naz A, Sultan M.T, Qayyum M.M. Anti-oncogenic perspectives of spices/herbs: A comprehensive review. EXCLI journal. 2013;12:1043-65.
- Huang J, Yang B, Xiang T, Peng W, Qiu Z, Wan J, et al. Diallyl disulfide inhibits growth and metastatic potential of human triple-negative breast cancer cells through inactivation of the beta-catenin signaling pathway. Molecular nutrition & food research. 2015;59(6):1063-75.
CrossRef - Kim S, Lee H.G, Park S.A, Kundu J.K, Keum Y.S, Cha Y.N, et al. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PloS one. 2014;9(1):e85984.
CrossRef - Yin X, Zhang R, Feng C, Zhang J, Liu D, Xu K, et al. Diallyl disulfide induces G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK pathways in human esophageal squamous cell carcinoma. Oncology reports. 2014;32(4):1748-56.
CrossRef - Singh S.V, Mohan R.R, Agarwal R, Benson P.J, Hu X, Rudy M.A, et al. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochemical and biophysical research communications. 1996;225(2):660-5.
CrossRef - Lan X, Sun H, Liu J, Lin Y, Zhu Z, Han X, et al. Effects of garlic oil on pancreatic cancer cells. Asian Pacific journal of cancer prevention : APJCP. 2013;14(10):5905-10.
CrossRef - Tsubura A, Lai Y.C, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anti-cancer agents in medicinal chemistry. 2011;11(3):249-53.
CrossRef - Madhunapantula S.V, Desai D, Sharma A, Huh S.J, Amin S, Robertson G.P. PBISe, a novel selenium-containing drug for the treatment of malignant melanoma. Mol Cancer Ther. 2008;7(5):1297-308.
CrossRef - Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute. 1990;82(13):1107-12.
CrossRef - Shailasree S, Venkataramana M, Niranjana S.R, Prakash H.S. Cytotoxic effect of p-Coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Molecular neurobiology. 2015;51(1):119-30.
CrossRef - Strober W. Trypan blue exclusion test of cell viability. Current protocols in immunology. Appendix 3:Appendix 3B. 2001.
- Kumar S.R, Rajkapoor B, Perumal P, Dhanasekaran T,Jose A.M, Jothimanivannan C. Antitumor Activity of Prosopis glandulosa Torr. on Ehrlich Ascites Carcinoma (EAC) Tumor Bearing Mice. Iranian journal of pharmaceutical research. IJPR. 2011;10(3):505-10.
- Sharma S.M, Mohan M, Kumari S, Sorake S.J. Evaluation of glutathione in oral squamous cell carcinoma. Journal of maxillofacial and oral surgery. 2009;8(3):270-4.
CrossRef - Sun Y, Oberley L.W, Li Y. A simple method for clinical assay of superoxide dismutase. Clinical chemistry. 1988;34(3):497-500.
- Sheng Y, Abreu I.A, Cabelli D.E, Maroney M.J, Miller A.F, Teixeira M, et al. Superoxide dismutases and superoxide reductases. Chemical reviews. 2014;114(7):3854-918.
CrossRef - Aquilano K, Baldelli S, Ciriolo M.R. Glutathione: new roles in redox signaling for an old antioxidant. Frontiers in pharmacology. 2014;5:196.
CrossRef - Jaganathan S.K, Mondhe D, Wani Z.A, Pal H.C, Mandal M. Effect of honey and eugenol on Ehrlich ascites and solid carcinoma. Journal of biomedicine & biotechnology. 2010;2010:989163.
CrossRef - Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food and chemical toxicology. an international journal published for the British Industrial Biological Research Association. 2013;57:362-70.
CrossRef - Li Y, Li S, Meng X, Gan R.Y, Zhang J.J, Li H.B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients. 2017;9(7).
CrossRef - Park E.K, Kwon K.B, Park K.I, Park B.H, Jhee E.C. Role of Ca(2+) in diallyl disulfide-induced apoptotic cell death of HCT-15 cells. Experimental & molecular medicine. 2002;34(3):250-7.
CrossRef - Oh E.T, Park H.J. Implications of NQO1 in cancer therapy. BMB reports. 2015;48(11):609-17.
CrossRef - Pink J.J, Wuerzberger-Davis S, Tagliarino C, Planchon S.M, Yang X, Froelich C.J, et al. Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during beta-lapachone-mediated apoptosis. Experimental cell research. 2000;255(2):144-55.
CrossRef - Feng C, Luo Y, Nian Y, Liu D, Yin X, Wu J, et al. Diallyl Disulfide Suppresses the Inflammation and Apoptosis Resistance Induced by DCA Through ROS and the NF-kappaB Signaling Pathway in Human Barrett’s Epithelial Cells. Inflammation. 2017;40(3):818-31.
CrossRef - Bottone F.G.J, Baek S.J, Nixon J.B, Eling T.E. Diallyl disulfide (DADS) induces the antitumorigenic NSAID-activated gene (NAG-1) by a p53-dependent mechanism in human colorectal HCT 116 cells. The Journal of nutrition. 2002;132(4):773-8.
CrossRef







