Manuscript accepted on :November 15, 2017
Published online on: --
Plagiarism Check: Yes
I. Made Pande Dwipayana1,2, Arini Junita1, Siswadi Semadi1, Ketut Suastika1, Made Ratna Saraswati1, Wira Gotera1 and Anak Agung Gede Budhiarta1
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Faculty of Medicine, Udayana University, Bali, Indonesia.
2Doctoral Program, Faculty of Medicine, Udayana University, Bali, Indonesia.
Corresponding Author E-mail: dwipayanapande@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1298
Abstract
Abdominal obesity is a part of metabolic syndrome. There are several causes of abdominal obesity and one of which is hormonal factor. Decrease of testosterone level has been thought as one of many possible cause of abdominal obesity in men, but the exact mechanism has not been well elaborated until now. The aim of this study was to evaluate the correlation of total testosterone and SHBG levels with abdominal obesity in Legian village, Kuta, Bali, Indonesia. An analytic cross sectional study has been conducted. The samples were selected from healthy men population in Legian village, with the total number of 80 subjects. Among 80 samples, the lowest total testosterone level is 1.38 nmol/L, and sex hormone – binding globulin (SHBG) is 1.53 nmol/L. The highest total testosterone level is 32.44 nmol/L and SHBG level 75.43 nmol/L. The means of total testosterone and SHBG levels were 16.60 nmol/L and 30.03 nmol/L respectively. We found significant negative correlation between abdominal obesity with testosterone levels (r=-0.233, p=0.037), but there were no significant correlation between sex hormone binding globuline levels with abdominal obesity (r=-0.042, p=0.720). This finding support the hypothesis that lower testosterone level maybe responsible for abdominal obesity in men.
Keywords
Abdominal Obesity SHBG; Total Testosterone;
Download this article as:| Copy the following to cite this article: Dwipayana I. M. P, Junita A, Semadi S, Suastika K, Saraswati M. R, Gotera W, Budhiarta A. A. G. Correlation of Total Testosterone and Sex Hormone Binding Globuline Level with Abdominal Obesity in Male Population of Legian Village, Kuta, Bali, Indonesia. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Dwipayana I. M. P, Junita A, Semadi S, Suastika K, Saraswati M. R, Gotera W, Budhiarta A. A. G. Correlation of Total Testosterone and Sex Hormone Binding Globuline Level with Abdominal Obesity in Male Population of Legian Village, Kuta, Bali, Indonesia. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17995 |
Introduction
Metabolic syndrome is a group of disorders (abdominal obesity, hypertrygliceridemia, hypocholesterol HDL-emia, hyperglycemia and hypertension. The core mechanism of this syndrome is insulin resistance that in which correlate with abdominal obesity. Waist circumference above normal range, known as abdominal obesity, is the main clue for metabolic abnormality related with insulin resistance, which correlates with increased risk of cardiovascular disease. Metabolic syndrome is also increases the risk of type II diabetes mellitus (T2DM). Therefore early prevention or treatment of metabolic syndrome is expected to be able to prevent T2DM and cardiovascular disease.1
Based on the National Health Survey in year 2001 with the cut off of body mass index (BMI) ≥ 30 kg/m2, the prevalence of obesity in men and women was 1.1% and 3.6%, whereas if using cut off ≥ 25 kg/m2, the prevalence of obesity in men and women was 8.1% and 17.1%.2 Apparently obesity is also a problem in rural area. Study in Purworejo, Indonesia, the prevalence of overweight and obese women was 13%,3 whereas the prevalence of obesity in Sangsit, Singaraja, Bali, Indonesia was 27.4%.4
The pathogenesis of the metabolic syndrome involves many factors, such as: obesity, less exercise, diet and other factors, as well as genetic.5 Several studies have shown levels of testosterone and sex hormone – binding globulin (SHBG) were lower in male associated with visceral obesity, insulin resistance or hyperinsulinemia and dyslipidemia. The relationship between testosterone and SHBG with decreased lipid profile is secondary to the accumulation of fat, but it seems an independent relationship between low testosterone levels with hyperinsulinemia and dyslipidemia. Low testosterone levels can also predict severe abdominal obesity and the development of diabetes mellitus in male. The relationship between the levels of dehidroepiandosterone with metabolic syndrome components in male is inconsistent, but some studies showed that dehidroepiandosterone levels is associated with the failure of glucose tolerance and insulin resistance. Testosterone itself is holding a major role in the pathogenesis of the metabolic syndrome since it is a component that build the syndrome itself, by decreasing muscle mass, increase abdominal obesity and decrease insulin sensitivity. The effects of weight loss on total and free testosterone level and also SHBG still cannot be explained well, but overall, abdominal obesity increases the glucocorticoid production and replacement, resulting in disruption of control axis hypothalamic-pituitary-adrenal and may cause slight hypoandrogenism in male.6 Only few population based studies assessed the relationship between testosterone and SHBG with abdominal obesity.
Study in Sangsit, Singaraja, Bali in 2004 found the prevalence of abdominal obesity in male was 10.8% and metabolic syndrome was 17.2%, by using NCEP-ATP III criteria. Study in Ubud, Bali in 2007 among 160 adult male found the prevalence of abdominal obesity was 51.88%.4 Overall, the prevalence of abdominal obesity is higher in urban than in rural area. Legian is a village located in the district of Kuta, Bali, Indonesia. Settlers and the indigenous people live together in this area, so this area is one of the urban area in Bali. Study on the relationship of testosterone and SHBG levels with abdominal obesity has never been done in Bali. Therefore the study was conducted to determine the correlation between testosterone and SHBG levels with abdominal obesity among the male population in Legian, Kuta, Bali, Indonesia.
Material and Method
This study was a cross-sectional study. This study was approved by local ethic committee. The target population was adult male. Accessible population was adult men in Legian who are willing to undergo the study. Patients with chronic and acute diseases as well as using some medications that affect the levels of total testosterone and SHBG were excluded.
The sample size was calculated based on a single sample formula for population, found as many as 80 subjects. Stratified random sampling was used to get the samples. Anamnesis, physical examination, basic laboratory tests (fasting and 2 hours post prandial blood sugar, insulin, serum creatinin, AST, ALT, lipid profile) and specific laboratory test (total testosterone and SHBG) were conducted. Descriptive analysis and Spearman test were used as statistical analysis.
Results
From 80 subjects, 73 subjects found with abdominal obesity and 7 subjects with no abdominal obesity. Mean of age was 46.41 ± 9.67 years, with mean of abdominal circumference was 97.75 ± 8.90 cm. The range of total testosterone level was 1.38 – 32.44 nmol/L, with mean was 16.61 ± 5.27 µIU/mL. The subject characteristic can be seen in table 1.
Table 1: Subjects characteristic
| Variable | Min | Max | Mean | K-S Test |
| Age (years) | 24 | 73 | 46.41 ± 9.67 | p < 0.05 |
| Weight (kg) | 54.70 | 126.20 | 79.71 ± 12.56 | p < 0.05 |
| Height (cm) | 129.00 | 181.00 | 167.33 ± 8.27 | p < 0.05 |
| Abdominal circumference (cm) | 62.50 | 128.00 | 97.75 ± 8.90 | p < 0.05 |
| BMI (kg/m2) | 20.84 | 45.92 | 28.51 ± 4.39 | p < 0.05 |
| Insulin level (µIU/mL) | 1.90 | 24.50 | 7.38 ± 4.87 | p < 0.05 |
| Fasting blood glucose (mg %) | 74.0 | 344.00 | 101.84 ± 42.06 | p < 0.05 |
| Testosterone level (nmol/l) | 1.38 | 32.44 | 16.61 ± 5.27 | p < 0.05 |
| SHBG level (nmol/l) | 1.53 | 75.34 | 30.03 ± 15.87 | p > 0.05 |
| 2 hours post prandial blood glucose (mg%) | 72 | 581.00 | 117.86 ± 70.98 | p < 0.05 |
K-S: Kolmogorov – Smirnov, p > 0.05 = normal distribution
There are some factors affect the total testosterone level and SHBG in this study, i.e. smoking, hypertension (systolic blood pressure ≥ 140 mmHg and or diastolic blood pressure ≥ 90 mmHg) and diabetes. The subjects with smoking habit was 24 (30%), DM 7 subjects (10.81%), and hypertension 12 subjects (16.22%).
Table 2: Proportion of some variables
| Variable | N | Percent (%) |
| 1. Smoking
– Smoker – Non smoker |
24 56 |
30 70 |
| 2. Blood pressure
– Hypertension – No hypertension |
15 65 |
18.75 81.25 |
| 3. Blood glucose
– DM – non DM |
8 72 |
10 90 |
| 4. Creatinin clearance
– 30 – ≥ 90 ml/min – 15 – 29 ml/min – < 15 ml/min |
80 0 0 |
100 0 0 |
The parameter of abdominal obesity is waist circumference. There were negative significant correlation between total testosterone level and waist circumference (r=-0.233; p=0.037) and positive significant correlation between fasting insulin level and waist circumference (r=0.315; p=0.004). SHBG and other variables were not significantly correlated with waist circumference (table 3).
Table 3: Spearman rho correlation test
| Variable | Waist circumference | |
| R | p | |
| Age (years) | 0.059 | 0.604 |
| Systolic blood pressure (mmHg) | 0.197 | 0.080 |
| Diastolic blood pressure (mmHg) | 0.202 | 0.073 |
| Fasting blood sugar (mg%) | 0.059 | 0.604 |
| 2 hours post prandial blood sugar (mg%) | 0.135 | 0.244 |
| Insulin | 0.315 | 0.004* |
| Serum Creatinin (mg/dL) | 0.017 | 0.881 |
| SHBG (nmol) | 0.010 | 0.927 |
| Testosterone (nmol) | -0.233 | 0.037* |
* p<0.05= significant.
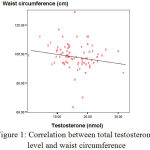 |
Figure 1: Correlation between total testosterone level and waist circumference
|
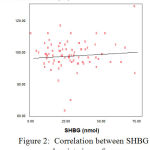 |
Figure 2: Correlation between SHB and waist circumference
|
Discussion
Abdominal obesity is found in the earliest phase of metabolic syndrome. In this study, we found the prevalence of abdominal obesity is 91.25 % with IDF criteria (abdominal obesity in male if the waist circumference is ≥ 90 cm). There are several factors that influence the occurrence of abdominal obesity, one of which is a hormonal factors. In male population, total and bioavailable testosterone level, SHBG and dehydroepiandrosterone sulfate (DHEA-S) are related with some unfavorable cardiovascular risk factors, i.e. lipid and blood pressure, which are component of metabolic syndrome and insulin level.7 In this study, bioavailable testosterone level and DHEA-S were not measured because of the limitations of resources.
In morbid obese men and men with insulin resistance are often found a low level of testosterone and an increase of estradiol level. Relationship of total plasma testosterone level and insulin sensitivity in men is mediated by body fat distribution. Low plasma testosterone level and central obesity are related with the change of carbohydrate and fat metabolism.8 In this study, we found significantly negative correlation between total testosterone and abdominal obesity. The same results also found by Hall, et al. in their study of 1822 subjects and concluded that abdominal obesity is a factor related with low level of testosterone (increase every 10 cm of waist circumference; OR = 1.75; 95% CI = 1.45 – 2.12).9
Obesity is associated with total, bioavailable and free testosterone level, and SHBG. Some studies have shown the inverse relationship between total and free testosterone level with degree of visceral fat which is measured by waist circumference and CT scan. Ratio of testosterone-estradiol decrease in male with obesity, shows that lower testosterone level as result of increase aromatase activity which metabolizes testosterone into estradiol. Concentration and activity of aromatase is related with adipose tissue number. Aromatase affects visceral fat more signifficant compare to subcutaneus fat. Degree of obesity is directly and proportionaly affects the metabolism of testosterone into estradiol. Homeostatic response of hypotalamus-pituitary-testicular axis up to the decline in testosterone is attenuated by inhibiting effects due to higher estradiol levels, leptin resistance and suppression of pro-inflammatory adipositokine.10
In this study, we found no significant correlation between SHBG level and abdominal obesity. Laaksonen, et al. reported that low level of total and free testosterone and SHBG had strong relationship with metabolic syndrome. Low SHBG level indirectly predicts high level of free testosterone. However, the mechanism that correlates low level of SHBG with metabolic syndrome still unclear and still need further investigation.11
Conclusion
From this study we can conclude that:
Total testosterone level has a negatif correlation with abdominal obesity in male population of Legian Village.
SHBG level has no correlation with abdominal obesity in male population of Legian Village.
Acknowledment
The author declare no conflict of interest regarding this study
Conflict of Interest
No conflict of interest reported
This research funded by Division of Endocrinology and Metabolism, Department of Internal Medicine, Faculty of Medicine, Udayana University, Sanglah General Hospital, Denpasar, Bali, Indonesia
References
- Syahbuddin S. Metformin pencegahan DM tipe 2 (komplikasi kardiovaskular). Proceeding The MetS-The 3rd stage of Obesity: Prevention and Treatment. 2007.
- Soemantri S., Pradono J., Hapsari D. National housnoehold health survey morbidity study-NCD risk factors in Indonesia. Available at http://www.who.int/chp/steps/STEPS_Report_Indonesia_National_2001.pdf (accessed on 16th July 2005). 2001.
- Nawi N. g., Stenlund H., Bonita R., Hakimi M., Wall S., Weinehall L. Preventable risk factors for noncommunicable diseases in rural Indonesia prevalence study using WHO steps approach. Bull World Health Organ. 2006;84:305-13.
CrossRef - Suastika K., Budhiarta A. A., Sutanegara I. N., Aryana I. G. P. S., Saraswati I. M. R., Gotera W., et al. Epidemiology study of metabolic syndrome in rural population in Bali. Int J Obes. 2004;28:s55-9.
- Al-Delaimy W. K., Willett W. C., Manson J., Speizer F. E., Hu F. B. Smoking and mortality among women with type 2 diabetes the nurses health study cohort. Diabetes Care. 2001;24:2043-8
CrossRef - Federman D. D. The biology of human sex differences. N Engl J Med. 2006;354(14):1507-14.
CrossRef - Muller M., Grobbee D. E., den Tonkelaar I., Lamberts S. W., van der Schouw Y. T. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90(5):2618-23.
CrossRef - Simon D., Charles M., Nahoul K., Kremski J., Hully V., Joubert E., et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men the Telecom Study. J Clin Endocrinol Metab. 1997;82(2):682-5.
CrossRef - Hall S. A., Esche G. R., Araujo A. B., Travison T. G., Clark R. V., Williams R. E., et al. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab. 2008;93:3870-7.
CrossRef - Jones T. H. Obesity metabolic syndrome, and diabetes. In: Jones H, editor. Testosterone deficiency in men. Oxford: Oxford University Press. 2008:107-19.
CrossRef - Makhsida N., Shah J., Yan G., Fisch H., Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol. 2005;174:827-34.
CrossRef







