Manuscript accepted on :November 29, 2017
Published online on: --
Plagiarism Check: Yes
Omnia Ahmed Mohamed Abd El-Gaphar1 , Amira Mourad Abo-youssef2
, Amira Mourad Abo-youssef2 and Gouda Kamel Helal3
and Gouda Kamel Helal3
1Pharmacology and Toxicology Department, Faculty of Pharmacy, Nahda University, Egypt.
2Pharmacology and Toxicology Department, Faculty of Pharmacy, Beni-Sueif University, Egypt.
3Pharmacology and Toxicology Department, Faculty of Pharmacy, Al-Azhar University, Egypt.
Corresponding Author E-mail: Omneaahmed25@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1329
Abstract
The Janus kinase-signal transducers and activators of transcription (JAK/STAT) is one of signaling pathways that mediate rheumatoid arthritis (RA) pathogenesis. Present study designed to evaluate the stimulatory role of lipopolysaccharide (LPS) JAK2/STAT3-induced RA, compared to the traditional Complete Freund's Adjuvant (CFA)-induced RA. Rats were allocated into normal control group (saline only), CFA arthritic control groups (0.4 ml/3days/12days, S.C.), and LPS group (100µg/kg/day/12days, I.P.). After 12 days of induction, tissue samples were collected for assessment of tissue phosphorylated JAK2 and STAT3, while serum samples used for biochemical evaluation of interleukin-6 (IL-6) and anti-citrullinated protein antibody (ACPA). Results indicated that LPS significantly increased tissue phosphorylation of JAK2 and STAT3 in cope with significant increase in serum IL-6 and ACPA as compared to CFA. Conclusively, LPS can consider as a formidable inducer for RA, in partly through activation of JAK2/STAT3 signaling pathway, this may add a new approach for experimental induction of RA.
Keywords
Rheumatoid Arthritis; Lipopolysaccaride; JAK2; STAT3
Download this article as:| Copy the following to cite this article: El-Gaphar O. A. M. A, Abo-Youssef A. M, Helal G. K. Bacterial Lipopolysaccharide Vigorously Activate JAK2/STAT3 Induced Rheumatoid Arthritis Collate with Complete Freund's Adjuvant in Experimental Rats. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: El-Gaphar O. A. M. A, Abo-Youssef A. M, Helal G. K. Bacterial Lipopolysaccharide Vigorously Activate JAK2/STAT3 Induced Rheumatoid Arthritis Collate with Complete Freund's Adjuvant in Experimental Rats. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17875 |
Introduction
Rheumatoid arthritis (RA) is a progressive autoimmune inflammatory musculoskeletal disease1 characterized by over production of two known antibodies – rheumatoid factor and the most sensitive, specific anti-citrullinated peptide antibody (ACPA).2 Pathophysiology of RA involves a complex network of inflammatory and immunity interacting pathways that finally mediate abnormal release of matrix metalloproteinases (MMPs) in synovium and promote joint damage.3
The janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is one of most intracellular signaling pathways that recently have confirmed to play an important role in RA pathogenesis.4 The JAK/STAT signal transduction pathway is mainly utilized by pro-inflammatory cytokine such as interleukin-6 (IL-6) that expressed abundantly in the synovial fluid and regulate gene expression and cellular activation, proliferation, and differentiation (Boyle et al., 2015). Briefly, binding of cytokine IL-6 to its receptor activates JAKs, protein tyrosine kinases, phosphorylation and to the recruitment, phosphorylation of STATs that subsequently dimerize and translocate to the nucleus, where they can activate transcription of the targeted genes6.
Adjuvant induced arthritis is the classical and most common model for studying the pathogenesis of experimental RA in rats.7 Previous studies have shown that subcutaneous injection of mycobacterium tuberculosis in rats can massively activate pro-inflammatory cytokines release particularly IL-6 that has a pivotal role in activation of JAK1/STAT3-induced RA and cartilage degradation.8
Lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria, is a potent activator of a variety of immunity and inflammatory cascades9. LPS has been shown to initiate multiple intracellular signaling events, activate immune system and mediate the release of diverse pro-inflammatory mediators, including IL-6.10 Therefore, LPS has an imperative role in occurrence of some autoimmune and inflammatory diseases.11
The present study, therefore, was designed to evaluate and compare possible stimulatory effect of LPS on JAK2/STAT3 signaling pathway and subsequently its prospect role in RA pathogenesis versus the classical CFA-induced arthritis.
Materials and Methods
Animals
Adult female Wister Albino rats, weighing 180–200 g were obtained from Modern Veterinary Office for Laboratory Animals, Cairo, Egypt. Rats were adapted in plastic cages with free access to food and water for two weeks before being subjected to laboratory experiments. Rats were maintained in a room controlled at (25 ± 0.5°C) and relative humidity (55 ± 1) with a 12 h light–dark cycle. All experiments and animal care procedures were approved by the guidelines of Beni-Sueif Animal House approved by the Pharmacology and Toxicology Department, Faculty of Pharmacy, Beni-Sueif University, which based on the guidelines suggested by the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (Publication No. 80-23, revised 1978).
Drugs, Chemicals and Kits
Complete Freund’s Adjuvant from dried Mycobacteria and LPS (Escherichia coli serotype 0111:B4) were purchased from Sigma-Aldrich Chemical Company (Mosby, USA). Quantikine Enzyme-Linked Immunosorbent Assay (ELISA) Kit for serum IL-6 (Catalog No. R6000B) was from R&D Systems Europe Company, Ltd (United Kingdom, Europe). Anti-Cyclic Cirtullinated Peptide Antibody (CCPAb) (Catalog No. MBS7240750) ELISA Kits were obtained from MyBiosource Company (California, USA).
Polyclonal antibodies against JAK2 (Catalog No. 44-426G) and STAT3 (Catalog No. 44-380G) for western blot analysis were obtained from Thermo Fisher Scientific INC (Massachusetts, USA). RIBA lysis buffer PL005 and Bradford Protein Assay Kit (Catalog No. SK3041) for quantitative protein analysis were provided by Bio BASIC INC. (Marhham Ontario, Canada), TGX Stain-Free™ FastCast™ Acrylamide Kit (SDS-PAGE) (Catalog No. 1610185) was provided by Bio-Rad Laboratories (TNC, USA).
Experimental Design
Animals were randomly assigned into three groups; a normal control group (received vehicle only), a CFA arthritic control group and LPS group. CFA was given by subcutaneous injection (S.C.) in a dose of 0.4 ml on 1, 5 and 9 days. LPS was given by intra-peritoneal (I.P.) injection at a dose of 100µg/kg from day 1 through 12 days. Doses of CFA and LPS had been chosen based on pilot trials guided with published literature (Omnia et al. 2015) and (Fortier et al. 2007) respectively. On day 13 serum and tissue samples were taken for assessment of serum IL-6 as a specific ligand for JAK/STAT pathway and serum ACPA as a specific rheumatoid biomarker, as well as, tissue expression of JAK2 and STAT3.
Methods
Induction of Rheumatoid Arthritis
For RA induction, three separate S.C doses of CFA, each of 0.4 ml were injected in planter surface of the right hind limb, on days 1, 5 and 9.this study relied on aggressive modified model of arthritis (Omnia et al. 2015).
Serum Sampling
Twenty-four hours following the last dose of CFA and LPS, animals were deeply anesthetized and blood samples were collected into non heparinized tubes from orbital venous plexus and centrifuged at 1000 xg for 30 minutes in a cooling (Sigma 3-30k, USA). The clear serum layer was withdrawn and stored in a -80C deep freezer (Als Angelantoni Life Science, Italy) for further assessment of serum IL-6 and ACPA.
Tissue Sampling
Soon after blood withdrawal, animals were scarified and the fresh whole knee joints were dissected, placed in round-bottom microcentrifuge tubes and kept on ice for immediate homogenization for western blot analysis of JAK2 and STAT3.
Assessment of Serum IL-6 and ACPA Biomarkers
Serum IL-6 and ACPA were assessed using ELISA reagent kits according to the methods described by kits manufacturer instructions based on the principles described earlier (Engvall et al., 1971; Lequin 2005). Both ELISA assays were done using Awareness Technologies Stat Fax 2100 Microplate Reader (SKU#: 8036-10-0020).
Western Blot Analysis for Tissue Level of JAK2 and STAT3
Tissue levels of JAK and STAT were measured using the Western blot technique described earlier.16,17 To ascertain the phosphorylation level of the various JAK and STAT proteins in synovial tissue, briefly, Each 5mg of synovial tissue was treated with 300 µl RIPA lysis buffer and homogenized with electrical homogenize and maintained on a constant agitation for 2 h at 4°C, then the lysate was kept on ice for 30 minutes and Cell debris was removed by centrifugal ion at ~16,000 xg for 30 minutes at 4°C. The protein concentrations were determined by Bradford assay, separated by 10% SDS-PAGE using BioRad mini protein electrophoresis separation unit (USPTO) and transferred onto a nitrocellulose membrane. After blocking with tris-buffered saline with Tween 20 (TBST) buffer and 3% bovine serum albumin (BSA) at room temperature for 1 hr, the membranes were incubated overnight with primary antibodies against p-JAK2 and p- STAT3, followed with appropriate horseradish peroxidase conjugated secondary antibodies. The chemiluminescent substrate (ClarityTM Western ECL substrate – BIO-RAD, USA) was applied to the blot according to the manufacturer’s recommendation. The chemiluminescent signals were captured using a CCD camera-based imager. Image analysis software was used to read the band intensity of the target proteins against control sample after normalization by beta actin (B-actin) on the ChemiDoc Tm MP imaging System with Image Lab Tm Software, version 5.1 (Bio-Rad, 170-8280).
Statistical Analysis
Statistical analysis were performed by one way analysis of variance (ANOVA) followed by Tukey-Kramer post hoc test, computer software program (Graph Pad Prism 6) with a value of P < 0.05 considered statistically significant. Results were expressed as means ± standard error of the mean (S.E.M).
Results
Effect of CFA and LPS on Tissue JAK2 and STAT3 Expression
Figure 1 (A) showed that both CFA and LPS significantly activate JAK2 tissue expression if compared to normal control group. No significant difference between each other was observed. Figure 1 (B) illustrated that rats injected with CFA or LPS significantly increased expression of STAT3 in synovium tissue when compared to normal rats. No significant difference in tissue level of STAT3 had been detected between CFA-rats and LPS-rats.
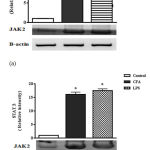 |
Figure 1: Effect of CFA and LPS on tissue JAK2 and STAT3 expression.
|
Each value represents the mean of 10 experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-keuls multiple comparisons test. a Significantly different from control group at p < 0.05. b Significantly different from CFA arthritis control group at P < 0.05. c Significantly different from LPS group at P < 0.05. CFA: Complete Freund’s Adjuvant; LPS: Lipopolysaccharide; JAK: Janus kinase; STAT: Signal transducers and activators of transcription; SEM: standard error of the mean.
Effect of CFA and LPS on Serum Inflammatory Cytokine IL-6 and ACPA
As shown in Table 1, the concentration of serum cytokine IL-6 was significantly elevated in CFA group and LPS if compared with that of normal group. No significant difference has been shown between CFA and LPS groups. Moreover, both CFA group and LPS group induced an elevation in serum ACPA if compared to normal control, with non-obvious significant different between each group.
Table 1: Effect of CFA and LPS on serum IL-6 and ACPA.
| Parameters
Groups |
IL-6
Pg/ml |
ACPA
ng/ml |
| Normal Control | 22.57 ± 1.56 | 0.261 ± 0.01 |
| CFA | 127.9 ± 7.42 a | 23.02 ± 0.43 a |
| LPS | 123.9 ± 8.38 a | 27.61 ± 1.59 a |
| CFA + LPS | 176.9 ± 6.11 a,b,c | 39.22 ± 1.93 a,b,c |
Each value represents the mean of 10 experimental rats ± SEM. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-keuls multiple comparisons test. a Significantly different from control group at p < 0.05. b Significantly different from CFA arthritis control group at P < 0.05. c Significantly different from LPS group at P < 0.05. CFA: Complete Freund’s Adjuvant; LPS: Lipopolysaccharide; ACPA: Anticitrullinated protein antibody; IL-6: Interleukin-6; SEM: standard error of the mean.
Discussion
Rheumatoid arthritis is one of the complex inflammatory, immune-mediated diseases, which depend on multiple signaling and effector pathways that ultimately leads to the progressive destruction of joint structure.
Interleukin-6 is one of the key cytokines that drive immune response, particularly during inflammatory joint. Previously, it was evidenced that IL-6 use the common receptor subunit gp130 for activation of JAK2/STAT3 signaling pathway, consequently promote physiological responses and molecular mechanisms involved in RA pathogenesis.
It has been reported that endotoxemia was adjunct with inflammation as well as increase in IL-6 concentration.18 Depending on that, the present study investigate the possible inducible role of LPS, the prototypical endotoxin, on JAK2/STAT3 signaling pathway activation in RA compared with the classical CFA-induced RA in rats.
The current study results clarified that three S.C injected doses of CFA could positively activate JAK2/STAT3 signaling pathway in synovium tissue through releasing of inflammatory cytokine IL-6, this was evidenced by significant increase in serum IL-6, tissue JAK2 and tissue STAT3 compared to normal rats. In support, adenovirus-mediated gene transfer of CIS3 could suppress bone destruction and ameliorate IL-6-gp130-JAK2-STAT3–signaling pathway induced by CFA.19 Moreover, CFA showed an extensive joint destruction and arthritis progression, confirmed by significant elevation in serum ACPA. Similarly, it has previously reported by Ali et al. (2015) and Bonezzi et al. (2016) that CFA is one the most appropriate agents for experimental induction of RA.
It seems from our results that I.P injection of LPS to rats had a stimulatory effect on both JAK2 and STAT3. Despite other investigations had not discuss role of LPS in activation of JAK/STAT pathway in RA, many of them handled its role in other disease where Victoni et al. (2014) and Ho et al. (2017) reported that LPS can induce JAK/STAT activation in bronchial airway and prostate cells respectively. This activation of JAK2/STAT3 signaling pathway with LPS can be explained to the significant increase in serum IL-6 after LPS injection to rats, where LPS interact with host cells, such as monocytes and macrophages, and leads to the production of inflammatory mediators, including IL-6.24
To clarify whether this activation of JAK2 and STAT3 can play a precise role in RA development, ACPA, highly specific and sensitive marker for RA,25 was measured. Our results viewed that LPS could trigger RA progression concomitant with harmful joint damage, evidenced by significant increase in in serum ACPA if compared to normal group. Almost no researches were conducted regarding the effect of LPS on ACPA in experimental arthritis, however Shi et al. (2014) illustrated that LPS can induce immunopathological cascades that could contributed to some autoimmune disease. Side by side, Le et al. (2013) stated that LPS can increase the formation and survival of mature osteoclasts. Our data distinct that LPS exhibited a motivated effect on each of; tissue JAK2, STAT3, serum IL-6 and serum ACPA at the same level as CFA or even little more. Putting together, these findings strongly suggest that LPS can induce RA at least in part due to stimulation of intracellular signal JAK/STAT/MMP-1 pathway. Additionally, those inspire us to suppose that LPS could produce a more and faster drastic effect in RA progression than do CFA.
Conclusion
Conclusively, according to the results of the present investigation, it is clearly obvious that LPS that was never used earlier as a model for RA induction can be a perfect model for experimental induction of RA at least in part through stimulation of JAK2/STAT3 signaling pathway.
Acknowledgments
The authors are grateful to Molecular Biology Lab, Faculty of Medicine, Cairo University, Egypt for biochemical analysis of some parameters.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Singh J. A., et al. American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. ARTHRITIS Rheumatol. 2016;68:1–26.
CrossRef - Soroush M., Mahmoudi M & Akhlaghi M. Determination of Specificity and Sensitivity of Rheumatoid Factor and Anti CCP Tests in Patients with RA in Private Clinic in Tehran Iran. Biomed. Pharmacol. J. 2016;9:775–780.
CrossRef - Chen Y., Nixon N. B., Dawes P. T & Mattey D. L. Influence of variations across the MMP-1 and -3 genes on the serum levels of MMP-1 and -3 and disease activity in rheumatoid arthritis. Genes Immun. 2012;13:29–37.
CrossRef - Junttila I., Vidqvist K., Korpela M & Silvennoinen O. The activity of JAK-STAT pathways in rheumatoid arthritis : constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology. 2014;53:1–11.
- Boyle D. L. et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann. Rheum. Dis. 2015;1–6. doi:10.1136/annrheumdis-2014-206028
CrossRef - Mcinnes I. B & Schett G. Series Targeted treatments for rheumatoid arthritis 1 Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337.
CrossRef - Saravanan S., Islam V. I. H., Babu N. P., Pandikumar P & Thirugnanasambantham K. Swertiamarin attenuates inflammation mediators via modulating NF- j B / I j B and JAK2 / STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 2014;56:70–86.
CrossRef - Woon G., Na K., Lee R & Han R. IL-6 inhibitors for treatment of rheumatoid arthritis : past , present , and future. Arch. Pharm. Res. 2015;38:575–584.
CrossRef - Tan Y & Kagan J. C. Review A Cross-Disciplinary Perspective on the Innate Immune Responses to Bacterial Lipopolysaccharide. Mol. Cell. 2014;54:212–223.
CrossRef - Chi X., Ouyang X & Wang Y. ScienceDirect Hydrogen sulfide synergistically upregulates Porphyromonas gingivalis lipopolysaccharide- induced expression of IL-6 and IL-8 via NF- k B signalling in periodontal fibroblasts. Arch. Oral Biol. 2014;59:954–961.
CrossRef - Chen J., Szodoray P & Zeher M. Toll-Like Receptor Pathways in Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2016;50:1–17.
CrossRef - Omnia A., El -Gaphar A., Amira M., Abo-youssef A. A. A. Research Article Differential Effects of Atorvastatin and Prednisolone on Inflammation, Oxidative Stress and Hematological Biomarkers on Freund’s Adjuvant Induced-Arthritis in Rats 1. Int. J. Pharm. Sci. Rev. 2015;33:235–241.
- Fortier M., Luheshi G. N & Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. 2007;181:270–277.
- Engvall E., Jonsson K and Perlmann P. Antiserum , immunoglobulin fraction , and specific antibodies Iodinated proteins. Biochim. Biophys. Acta (BBA)-Protein Struct. 1971;251:427–434.
CrossRef - Lequin R. M. Enzyme Immunoassay ( EIA )/ Enzyme-Linked Immunosorbent Assay ( ELISA ). Clin. Chem. 2005;51:2415–2418.
CrossRef - Towbin H., Staehelint T & Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets : Procedure and some applications. Proc. Natl. Acad. Sci. 1979;76:4350–4354.
CrossRef - Burnette W. N. Electrophoretic Transfer of Proteins from “ Western Blotting ”: Sodium Dodecyl Sulfate-Polyacrylamide Gels to Unmodified Nitrocellulose and Radiographic Detection with Antibody and Radioiodinated Protein A. Anal. Biochem. 1981;112:195–203.
CrossRef - Chans E., Vidal H & Michalski M. Overfeeding increases postprandial endotoxemia in men : Inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol. Nutr. Food Res. 2014;58:1513–1518.
CrossRef - Shouda T. et al. Induction of the cytokine signal regulator SOCS3 / CIS3 as a therapeutic strategy for treating inflammatory arthritis. J. Clin. Invest. 2001;108:1745–1747.
CrossRef - Ali E. A. I., Barakat B. M & Hassan R. Antioxidant and Angiostatic Effect of Spirulina platensis Suspension in Complete Freund ’ s Adjuvant-Induced Arthritis in Rats. PLoS One. 2015;10:1–13.
- Bonezzi F. T. et al. An Important Role for N -Acylethanolamine Acid Amidase in the Complete Freund ’ s Adjuvant Rat Model of Arthritis s. ournal Pharmacol. Exp. Ther. 2016;356:656–663.
CrossRef - Victoni T. et al. Roflumilast N-Oxide Prevents Cytokine Secretion Induced by Cigarette Smoke Combined with LPS through JAK / STAT and ERK1 / 2 Inhibition in Airway Epithelial Cells. PLoS One. 2014;9:1–11.
CrossRef - Ho C. et al. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK / STAT-1 signaling pathway. PLoS One. 2017;12:1–19.
- Runkel F., Gokorsch S & Häberlein H. Ivy leaves dry extract EA 575 ® decreases LPS-induced IL-6 release from murine macrophages. Die Pharm. Int. J. Pharm. Sci. 2016;71:158–161.
- Pikwer M. et al. Parity influences the severity of ACPA- negative early rheumatoid arthritis : a cohort study based on the Swedish EIRA material. Arthritis Res. Ther. 2015;17:1–6.
CrossRef - Shi J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187.
CrossRef - Le B., Berthelot J., Maugars Y & Heymann D. Osteoclasts in RA: Diverse origins and functions. Jt. Bone Spine. 2013;80:586–591.
CrossRef







