Manuscript accepted on :August 10, 2017
Published online on: --
Plagiarism Check: Yes
Rithvik Ganesh1 and Iyanar Kannan2
1Rice Middle School, 8500 Gifford Dr, Plano, TX 75025, USA.
2Department of Microbiology Tagore Medical College and Hospital The Tamilnadu Dr. MGR Medical University Rathinamangalam, Chennai – 600127, India.
Corresponding Author E-Mail: dr.ikannan@tagoremch.com
DOI : https://dx.doi.org/10.13005/bpj/1257
Abstract
Alzheimer’s disease is a deadly form of dementia, and can greatly affect the way a person can think and behave. It is the sixth leading cause of death in the US alone. Clusters of Beta-Amyloid (Plaques) and twisted tangles of a protein called Tau (Tangles) are the primary cause of Alzheimer’s disease, as noted by Alois Alzheimer in 1906. Plaques and Tangles can block cell-to-cell signaling and disintegrate the cell transport system. The 3-dimensional structure of three drug targets, Beta-secretase 1, Cholinesterase, and Tau Protein kinase were retrieved from the RCSB PDB database. A total of 150 derivatives of Curcumin, Bacopaside IV, and Ginkgolide B were generated using the ACD ChemSketch software. These files were then converted to the Brookhaven protein data bank file using the OpenBabel software. Preliminary docking studies were then performed using the iGEMDOCK v2.0 software. All the prepared ligands were then tested for drug-likeliness properties using the DruLiTo and admetSAR softwares. Finally, compounds with good fit and drug likeliness were subjected to final docking with the AUTODOCK VINA software. In this study, the ligand with the name 8-(1-fluoro-2-methylpropan-2-yl)-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclononadecane-5,15,18-trione is found to be a good inhibitor of 3 well-known drug targets. This is an effective lead molecule that can be used in the treatment of Alzheimer’s disease. Plaques and Tangles are major causes for Alzheimer’s disease. The novel lead molecule identified in this study is an inhibitor of these virulence factors and thus can be effective in controlling Alzheimer’s disease.
Keywords
Alzheimer’s disease; Beta-secretase 1; Cholinesterase; Ginkgolide B derivatives Molecular docking; Tau Protein kinase;
Download this article as:| Copy the following to cite this article: Ganesh R, Kannan I. Molecular Docking Study of Certain Plant Alkaloid Derivatives as Inhibitors of Various Drug Targets of Alzheimer’s Disease. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Ganesh R, Kannan I. Molecular Docking Study of Certain Plant Alkaloid Derivatives as Inhibitors of Various Drug Targets of Alzheimer’s Disease. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16412 |
Introduction
Alzheimer’s disease, also known as senile dementia, is a deadly disease that can greatly affect a person’s thinking and behavioral actions. It is also the most common form of dementia, a general term for a significant decline in mental abilities that could affect everyday life.1 It is also a progressive disease, and are of three known forms viz. Alzheimer’s: Early-onset, which happens to a person under 65, which is a fairly rare case, late- onset, which occurs for people above 65, the most common form, and familial Alzheimer’s Disease, which occurs because of genetic reasons, which occurs in less than 1% of the patients.2
The characteristics of Alzheimer’s include overall brain shrinking due to fewer nerve cells and connections, and tiny proteins in nerve tissues called plaques and tangles, which are a prime suspect of the disease. The chief component of the plaques is beta-amyloid, while the chief component of tangles is the tau protein. Plaques are ‘sticky’ proteins and can build up between nerve cells, and can cause significant problems to overall learning and cause memory loss. Tangles can disintegrate the main cell transport system, eventually killing the cell.3 At present many effective drug targets that include Beta-secretase, cholinesterase,4 gamma-animobutyric acid5 are proposed. Effective inhibitors of the protein targets can be an effective drug to control Alzheimer’s disease.
Phytochemicals are chemicals from plants. Derivatives, different variations, of these phytochemicals can be good inhibitors with better drug-likeliness properties.6 The Indian Ayurvedic system includes ancient known plant-based remedies for controlling Alzheimer’s disease and its symptoms. Curcumin, from turmeric (Curcumin longa), a herbaceous perennial from the ginger family, has been noted to help decrease plaque deposition. Bacopaside IV, from Brahma (Bacopa monnieri), a bitter tasting creeper plant, noted to help memory and brain function. Ginkgolide B, from the Maidenhair Tree (Ginkgo biloba), the only living species of the division Ginkgophyta, is also known to help enhance memory.7
Insilico methods are computer-based methods widely used in the pharmacological field of science to help discover inhibitors with high binding capabilities with a protein target, drug-likeliness properties, and ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) clarification. This requires searching protein databases like RCSB PDB, Quantitative Structure Activity Analysis relationships (Predicting the activity of new compounds based solely on chemical structure), and computational molecular docking. In silico methods have been used to create a various inhibitors for a spectrum of diseases.8,9
Molecular Docking is an important method in molecular biology and computer-aided drug design. Ligand- protein docking helps predict the binding mode of a Ligand and Protein of known 3-dimensional structures. Other important types of docking include protein-protein and nucleic acid-protein docking.10
In this study, an attempt has been made to design inhibitors from the three phytochemicals ie., Curcumin, Bacopaside IV and Ginkgolide B, against the well-known drug targets Beta-Secretase, Cholinesterase, and the Tau protein kinase. This has been done using the insilico docking method.
Materials and Methods
Protein Target Preparation
The 3D structure of Beta-secretase 1 (BACE1), Cholinesterase, and Tau Protein kinase were retrieved from the RCSB PDB data-base (http://www.rcsb.org/pdb/home/home.do).11 Their PDB codes are 513V, 1ACJ and 1J1B respectively. They were saved as a Brookhaven protein data bank file.
Ligand Generation
The 2D structure of Curcumin, Bacopaside IV, and Gingkolide B were retrieved from the PubChem online database. 150 ligands based on the structure of these phytochemicals were generated from the ACD/Chemsketch Software.12 The generated ligands were then saved in the MDL Molfile format. The ligands were then converted to a PDB file format using the OpenBabel chemistry toolbox.13
Rapid Protein-Ligand Docking
Rapid-Screening preliminary docking was performed using the software iGEMDOCK version 2.1. iGEMDOCK is a Drug Design System for molecular docking and screening by BioXGEM labs. iGEMDOCK outputs hydrogen bond, electrostatic, and Van Der Waals forces. The average of 3 trials was performed for each docking combination, to ensure consistency. Each phytochemical was paired with a protein target in the following manner: Curcumin- Cholinesterase, Bacopaside- BACE1, and Gingkolide B – Tau. These trials were each docked with a population size set to 200, with 70 generations and 2 solutions. The post-docking tool was then used to find the docking poses and energy values.14
Drug-Likeliness Property Analysis
After Rapid-Screening preliminary docking, all of the compounds were first tested for drug-relevant properties based on Chris Lipinski’s Rule of 5, which is a set of criteria that helps evaluate drug likeliness and if has properties that increase it chances in being a likely orally active drug in the human body.
The Set of Criteria is
Fewer than 5 hydrogen bond donors
Fewer than 10 hydrogen bond acceptors
A molecular weight (in Daltons) less than 500
A partitioning coefficient logP of less than 5
No more than 1 of these rules can be violated. These properties were predicted using the DruLiTo software. The 14 compounds that satisfied these properties were then tested for other drug likeliness properties the admetSar software. admetSar gave a detailed profile of ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties by taking a SMILES input.15
Final Docking
Molecules that satisfied all the above properties were then subject to a final docking with all 3 protein targets using the AUTODOCK VINA docking tool. AUTODOCK VINA is a flexible molecular docking tool.16 Following the above phases, 5 generated ligands were identified. Based on these results, conclusions about possible drug candidates have been made. All the AUTODOCK docks were using the Lamarckian Genetic Algorithm.17
Results and Discussion
A total of 150 ligands were derived from Curcumin, Bacopaside IV, and Ginkgolide B. These were then converted to PDB format using the OpenBabel software. All of these then were subject to a preliminary dock using the iGEMDOCK software. 23 ligands with good fit then went on to further study. These were then tested for drug-relevant properties based on Chris Lipinski’s Rule of 5. The 14 satisfying ligands were then tested for ADMET properties. The 5 satisfying ligands were then taken into further docking studies with the AUTODOCK VINA software with all 3 of the protein targets. Based, on these results, the ligand with the overall lowest binding energy was then considered to be the best inhibitor.
Table 1 depicts the Lipinski’s Rule of 5 properties and other drug-like properties which shows that the selected compound has very good drug likeliness.
Table 1: Drug properties of the selected ligand
| Lipinski’s Rule of five | admetSAR results showing some important drug properties | ||||||
| Molecular Weight | logP partition coefficient | Hydrogen Bond Donors | Hydrogen Bond Acceptors | Mutagenic (AMES toxicity) | Carcinogenicity | Blood Brain Barrier | Human Intestinal Absorption |
| 442.13 | -0.493 | 3 | 10 | NO | NO | YES | YES |
Table 2 results show the energy values of the selected ligand. The selected ligand showed good binding affinity with all the drug targets selected in this study.
Table 2: iGEMDOCK results
| Trials | Tau protein |
| Binding Affinity (kcal/mol) | |
| Trial 1 | -146.95 |
| Trial 2 | -142.98 |
| Trial 3 | -147.15 |
| Average/Final | -145.69 |
Table 3 shows the values of binding affinity of the selected ligand for all the three drug targets. The ligand showed an excellent binding affinity for all the drug targets. This confirms that the selected ligand can be an effective inhibitor for all these drug targets. The Figure 1 shows the docking pose the selected ligand for all the three drug targets
Table 3: AUTODOCK VINA results
| Target protein | Binding Affinity (kcal/mol) | RMSD lower bound | RMSD upper bound |
| Cholinesterase | -13.5 | 0.935 | 2.06 |
| BACE1 | -14.2 | 0.918 | 2.06 |
| Tau | -15.0 | 1.224 | 4.238 |
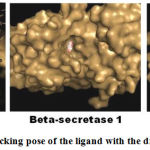 |
Figure 1: The docking pose of the ligand with the drug targets
|
Eventhough AD has a major health concern, there is no effective treatment approach in terms of its cure or prevention.18 The pharmacological therapy is largely symptomatic that imparts only the clinical benefits on cognitive and functional manifestations of the disease. The present strategy relies on the increase in the synaptic availability of acetylcholine to compensate the cholinergic deficit that arises from neuronal loss.19 This is done by inhibition of acetyl-cholinesterase by the drugs like donepezil, galantamine or rivastigmine.20 Now it is a well known fact that AD results from an increase in the accumulation of beta-amyloid protein and this forms the central event in the pathophysiology of AD.21,22 The beta-amyloid protein is formed by the cleavage of the amyloid precursor protein (APP) into smaller peptides. This event is mediated by the enzyme beta-secretase. Hence the inhibitors of beta-secretase can prevent the pathogenesis of AD.23 Thus beta-secretase is an effective drug target for the development of new drugs for AD. Another important drug target is Tau protein kinase. Tau is an alternatively spliced microtubule –binding protein the is predominantly expressed on the neurons.24 Apart from beta-amyloid plaques, the abnormal accumulation of tau leading to the formation of neurofibrillary tangles (NFTs) is considered to be important in pathophysiology of AD. The tau protein kinase is an important enzyme in the biosynthesis of Tau protein.25 Hence by the inhibition of this enzyme, the pathogenesis of AD can be prevented.
Thus in the present study, an attempt was made to find a lead compound that can bind to multiple drug target and thus can be effective in the treatment of AD. In this study, the compound 8-(1-fluoro-2-methylpropan-2-yl)-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclononadecane-5,15,18-trione derived from Ginkgolide B, a phytochemical present in Ginkgo Biloba shows excellent binding by docking studies to all the 3 well known drug targets. The proposed drug like compound can act as cholinesterase inhibitor, thus can be used as symptomatic drug used to improve the cognitive function by increasing the acetylcholine to the neuron. Further it can also inhibit beta-secretase and Tau protein kinase, and therefore can prevent the neurological damage and hence can form an effective pharmacological drug in the treatment of AD by preventing the further damage of neuron.
Conclusion
Clusters of Beta-Amyloid (Plaques) and twisted tangles of a protein called Tau (Tangles) are the primary cause of Alzheimer’s disease, as noted by Alois Alzheimer in 1906. The inhibition of these proteins and other known targets can help control Alzheimer’s disease. In this insilico study, with the help of molecular docking, a novel compound with name 8-(1-fluoro-2-methylpropan-2-yl)-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclononadecane-5,15,18-trione has been identified to inhibit 3 well known drug targets (Cholinesterase, BACE1, and Tau protein kinase). Thus, the inhibitor selected using the above procedures can act as an effective drug candidate to control Alzheimer’s disease.
Acknowledgments
The authors would like to thank Central Research Laboratory, Tagore Medical College, Chennai, India, in mentoring for the project. The authors would also like to thank Prof. M. Mala, Chairperson, Tagore group of Colleges, for providing necessary facilities for this project.
Conflict of Interest
None
References
- Maurer K., Volk S., Gerbaldo H. Auguste D and Alzheimer’s disease. Lancet. 1997;349(9064):1546-1549
CrossRef - Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168.
CrossRef - Armstrong R. A. b-amyloid plaques: stages in life history or independent origin? Dement Geriatr Cogn. Disord. 1998;9:227-238.
CrossRef - Drug development for Alzheimer’s disease: Where are we now and where are we headed? Am J Geriatr Pharmacother. 2009;7(3):167–185.
CrossRef - Rissman R. A., De Blas A. L., Armstrong D. M. GABA(A) receptors in aging and Alzheimer’s disease. J Neurochem. 2007;103(4):1285–1292.
CrossRef - Rammohan V. R., Descamps O., John V and Bredesen E. D. Ayurvedic Medicinal Plants for Alzheimer’s Disease: A Review. 2012;4(3):22.
- Schneider L. S. Ginkgo biloba extract and preventing Alzheimer disease. JAMA. 2008;300:2306–2308.
CrossRef - Raj L. S. M., Jude J., Kannan I., Krishna P. S., Shankar K. A. Molecular docking study for inhibitors of Aggregatibacter actinomycetamcomitans toxins in treatment of aggressive perioodontitis. Journal of Clinical and Diagnostic Research. 2014;9(11):ZC48 –ZC51.
- Jorgensen W. L. The many roles of computation in drug discovery. Science. 2004; 303(5665):1813–1818.
CrossRef - Cross J. B., Thompson D. C., Rai B. K., Baber J. C., Fan K. Y., Hu Y., Humblet C. Comparison of several molecular docking programs pose prediction and virtual screening accuracy. J Chem Inf Model. 2009;49(6):1455–1474.
CrossRef - Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H., Shindyalov I. N., Bourne P. E. The Protein Data Bank. Nucleic Acids Res. 20001;28(1):235-42.
- ACD/ChemSketch Freeware, version 10.00, Advanced Chemistry Development, Inc, Toronto, ON, Canada. 2012.
- O’Boyle M. N., Banck M., James A. C.,Morley C., Vandermeersch T ., Hutchison R. G. Open Babel: An Open Chemical Toolbox. Journal of Cheminformatics. 2011;3:33.
CrossRef - Yang J. M., Chen C. C. GEMDOCK: A generic evolutionary method for molecular Docking. Proteins. Structure, Function and Bioinformatics. 2004;55:288–304.
CrossRef - Cheng F., Li W., Zhou Y., Shen J., Wu Z., Liu G., Lee P. W., Tang Y. Admet SAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J Chem Inf Model. 2012;52(11):3099-105.
CrossRef - Trott O., Olson A. J. Auto Dock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multi threading. J. Comput. Chem. 2010;31:455-461.
- Garrett M. M. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998;19:1639–1662.
CrossRef - Aprahamian I., Stella F. F., Forlenza O. V. New treatment strategies for Alzheimer’s disease is there a hope? Indian J Med Res. 2013;138:449-460.
- Yiannopoulou G. K.,Papageorgiou G. S. Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord. 2013;6(1):19–33.
CrossRef - Scarpini E.,Schelterns P., Feldman H. Treatment of Alzheimer’s disease current status and new perspectives. The Lancet Neurology. 2003;2:539-547.
CrossRef - Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res. 2006;3:71-73.
CrossRef - Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110:1129-1134.
CrossRef - Hampel H. Current insights into the pathophysiology of Alzheimer’s disease: selecting targets for early therapeutic intervention. Int Psychogeriatr. 2012;24(1): S10-7.
CrossRef - Medeiros R., Baglietto-Vargas D., LaFerla F. M. CNS Neurosci Ther. 2011;17(5):514-524.
CrossRef - LaFerla F. M., Oddo S. Alzheimer’s disease: Abeta, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176.
CrossRef







