Manuscript accepted on :August 08, 2017
Published online on: --
Plagiarism Check: Yes
Khairun Nisa Berawi1, Liana Shidarti1, Samsu U. Nurdin2, Nur Indrawati Lipoeto3, Irza Wahid3, Jamsari5 and Endang Nurcahyani4
1Faculty of Medicine, University of Lampung, Bandar Lampung, Lampung, Indonesia.
2Department of Agricultural Technology, Faculty of Agriculture, University of Lampung,Bandar Lampung, Lampung, Indonesia.
3Faculty of Medicine, University of Andalas, Padang, West Sumatra, Indonesia.
4Department of Biology, Faculty of Mathematics and Natural of Sciences, University of Lampung, Bandar Lampung, Lampung, Indonesia.
5Faculty of Agriculture, University of Andalas, Padang, West Sumatra, Indonesia.
Corresponding Author E-mail: endang_nurcahyani@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1256
Abstract
Diabetes Mellitus (DM) is a degenerative disease that threatens global health. According World Health Organisation (WHO), Indonesia ranks 4th largest number of people with diabetes. Diabetes is a chronic metabolic disorder due to pancreas can not produce enough insulin or body can not use insulin, causing increasing blood sugar levels. The active ingredients like tannin, polyphenolic and acarbose in herbs have potential efectiveness as an antidiabetic and antioxidant.These herbs are being into alternative treatments that are often used in society as diabetic treatment. This study uses soursop leaves, bay leaves, and pegagan leaves extract. The leaves will be tested effectiveness as an antidiabetic and antioxidant with measuring effectiveness of enzymatic inhibition of α-amylase, α-glukosidase and total phenolic, that can affect blood sugar and oxidative stress conditions whiches experienced by patients with DM. Test result showedthat total phenol content obtained highest titer in soursop leavessingle extract, followed by a mixture of soursop leaves and pegagan leaves extract and lowest total phenol content obtained at a single extract of bayleaves. The highest potential effectiveness of anti-α-amylase similar with anti α-glucosidase obtained in a single extract of soursop leaves, followed by a mixture of soursop leaves and bay leaves extract and lowest potential was measured in single extractpegagan leaves and bay leaves. High effectiveness potential in herbs preparation as anti α-amylase, anti α-glucosidase, and antioxidant activity of total phenol content will treatand inhibit the progression of glucose metabolic disorder due to metabolic abnormalities in patients with DM.
Keywords
Anti α-amylase; anti α-glucosidase; antidiabetic; herbal mixture
Download this article as:| Copy the following to cite this article: Berawi K. N, Shidarti L, Nurdin S. U, Lipoeto N. I, Wahid I, Jamsari J, Nurcahyani E. Comparison Effectiveness of Antidiabetic Activity Extract Herbal Mixture of Soursop Leaves (Annona Muricata), Bay Leaves (Syzygium Polyanthum) and Pegagan Leaves (Centella Asiatica). Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Berawi K. N, Shidarti L, Nurdin S. U, Lipoeto N. I, Wahid I, Jamsari J, Nurcahyani E. Comparison Effectiveness of Antidiabetic Activity Extract Herbal Mixture of Soursop Leaves (Annona Muricata), Bay Leaves (Syzygium Polyanthum) and Pegagan Leaves (Centella Asiatica). Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16428 |
Introduction
Diabetes Mellitus is a degenerative disease which nowadays has become a threat to global health, including Indonesia. There were 382 million people living with diabetes in the world by 2013.1 In Indonesia an increase in the number of diabetics is more than 3% during period of 5 years (1995-2000) that put this country on ranked fourth among the 10 countries with the highest diabetes prevalence in the world after India, China and the USA.2 If in 2000 the prevalence of diabetes in Indonesia is estimated to be around 8.4%, then in 2030 this figure is expected to grow to 21.3%.3,2 Diabetes Mellitus is a disease characterized by the body’s inability to control blood sugar levels as a result of low production of insulin or the insensitivity of tissues to insulin in response to elevated blood sugar levels (insulin sensitivity). Diabetes can be classified into two categories, the first group (Type 1), insulin-dependent or juvenile/childhood-onset diabetes, is caused by the inability of the pancreas produce insulin and are usually caused by hereditary factors or damage to the immune system. In the second group (Type 2), non-insulin dependent or adult-onset diabetes, the pancreas is still able to produce insulin, but because of the low sensitivity, insulin is not able to push the course of glucose metabolism. In this group, the lifestyle of food and physical activity are major risk factors. It is common to all over the world with the proportion of patients achieving 90%, so prevention and treatment of type 2 diabetes is a top priority.4,5
Nutritional therapy is carried out against this disease aim to achieve and maintain blood sugar levels within the normal range, namely by balancing food intake with insulin availability or effectiveness of the use of highly influenced by food intake5. The method was taken to reduce elevated blood sugar after meals, it is inhibiting hydrolysis by the digestive enzymes of carbohydrate foods in the intestine.6 For this purpose, herbal medicine which has anti α-amylase activity or anti α-glucosidase widely used.6,7,8
The enzyme α-amylase and glucosidase can be inhibited by tannin or polyphenols.9,10 This compound is found in many plants used as antidiabetic traditional medicine or herbal medicine. Although there is no scientific data to support, but the empirical experience of society cemented position of traditional medicine in community. Advantages of the use of herbs for the prevention and treatment of diabetes include low doses of active ingredient that can be consumed regularly and more secure but has benefits because the content of the active components are mixed.
To maximize the efficacy of the herbal plants, mixing is a step that can be done. It should be known in advance whether the incorporation of herbs produces a synergistic effect or even antagonists.11 Synergistic or antagonistic effects of this mixture is very important information when the herbal plants will be developed as an antidiabetic drug or a functional food that is as guidelines for the prescription or formula. With this data it is possible to develop a more nutritious concoction and have good organoleptic quality by mixing various herbs and taste its antidiabetic efficacy in synergy.
Mechanism of action of herbal plants extract in inhibiting blood sugar increase are many way6.Several mechanisms are often used to assess the potential antidiabetic of a plant in vitro is the ability to inhibit the enzyme α-amylase and α-glucosidase.12 Plants or herbs that can inhibit the activity of these enzymes are considered able to inhibit starch digestion in the small intestine, thereby reducing the amount of glucose absorbed.
Oxidative stress plays an important role in the development of Diabetes Mellitus and its complications.13 An increase in free radical activity and a significant decrease in antioxidant status in animal models of diabetes 14,15 or diabetes15 due to increase blood sugar levels aggravate various metabolic disorders that occur in diabetics. Provision of antioxidant-rich foods can lower the oxidative stress and antioxidant status of diabetics increases.16 Therefore, in addition antiamylase activity and antiglucosidase, need to be studied also the mixing effect of antioxidant activity of the herbal plant mixture thereby increasing the effectiveness of herbal preparations as antidiabetic and antioxidant.
Diabetes Mellitus is a disease that not only bring harm to the patient but also for the family and the state.1 Therefore, getting the type of herbs that can work together in controlling blood sugar has a very high importance as it can be an alternative prevention and treatment are less expensive and safer for the public. The use of herbal plants in Indonesia as part of therapy of diabetics would be very advantageous because such materials have been used as traditional medicine, easily obtained from the surrounding environment can even be cultivated himself and proven to be safe allowing diabetics keep their blood sugar levels and prevent complications in up to the effectiveness of herbal preparations consumed.
To determine whether the mixing herbs produces a synergistic or antagonistic effect on this research will be testing activities and antiglucosidase antiamylase the herbal plant mixture.The in vitro method is a simplification of the in vivo method that aims to simplify and reduce the cost of testing a plant or herbal bioactive components. In vitro methods used in Phase 1 of this research is intended for screening in order to get a mix of the most potential herbal as an antidiabetic and antioxidant. Required testing on more complex models (in vivo) in order to ensure the effectiveness of the chosen mix of in vitro as well as to know the mechanism of action of the mixture in controlling the blood sugar of diabetics mellitus later.
The research objective is to assess the effect of mixing various herbs on the effectiveness of the mixture as antiamylase, antiglucosidase and antioxidants in vitro.
Material and Methods
Herbs are used in this study is pegagan leaves, soursop leaves, and bay leaves that will be purchased from local farmers in the area of Bandar Lampung, Lampung, Indonesia. Antidiabetic activity used to determine α-amylaseenzyme (α-amylase porcin) andα- glucosidase (from Saccharomyces cerevisiae). Other chemicals are required in this study was purchased from Sigma-Aldric (Germany). The tools used in this study is a shaker bath for the maceration process. Observations antienzym activity, antioxidant and total phenol using a microplate reader spectrophotometer.
Preparation of Extract and Mix Formulations
Herbs that will be used in the research will be purchased from local farmers in Bandar Lampung, Lampung, Indonesia. These materials after washing wind dried until the moisture content reaches around 12% (w/w). Furthermore, the dried material was crushed to obtain a coarse powder (not smooth)17.
Testing the Activity of Alpha-Amylase
Antiamylase activity is determined using the method used18 is based on the principle of iodine-starch test. A total of 40μl 0.02 M sodium phosphate buffer (pH 6.9), 0.02 units of enzyme α-amylase and 100 ml samples (0.0; 0.3; 0.6; 0.9; 1.2; 1.5 mg/ml) were mixed and incubated for 10 minutes at 37°C. Then 20 ml starch solution (1%, w/v) were added and incubated for 15 minutes at 37oC. The reaction was stopped by adding 20 ml of 1 M HCl followed by addition of 100 ml of a solution of iodine (I2.5mm and I 5.0mm). The color change was observed using spectrophotometer at a wavelength of 620 nm. As a control depicting enzyme activity of 100% is obtained from the treatment with a sample concentration of 0.0 mg/ml. IC50 (concentration required to reduce the 50% rate of reaction) was determined by observation of the yield curve.
Testing the Activity of Alpha-Glucosidase
Anti α-glucosidase activity is determined using the method of19 with some modifications. A sample of 100 ml with various concentrations (0.0; 0.3; 0.6; 0.9; 1.2; 1.5 mg/ml) were incubated with 50 ml of α-glucosidase from Saccharomycescerevisiae (0.1 U /ml) in 0.1 M phosphate buffer (pH 6.8) for 10 minutes at 37 °C. Subsequently 50μl substrate (5mm p-nitrophenyl-α-D glucopyranosida in 0.1 M phosphate buffer pH 6.8) was added to start the reaction. Kinetics of release of p-nitrophenol was measured using a spectrophotometer continuously for 5 minutes at intervals of 30 seconds at a wavelength of 405 nm. As standard is used akarbosa used as a standard. IC50 is determined based on the yield curve observations.
Total Phenol
Total phenol content is determined using the Folin-Denis reagent.20 A total of 10μl extract mixed with 790 ml of distilled water. Then added 50 ml of Folin-Denis reagent and homogenized. After 5 minutes, 150 ml of sodium carbonate (7.5%) was added and the mixture obtained was incubated at room temperature for 30 minutes. The absorbance was measured at a wavelength of 760 nm. The standards used are gallic acid and the results expressed as gallic acid equivalents.
Statistical Analysis
Experiments in the first year arranged in a Complete Randomized Block Design (CRBD) respectively with 3 and 8 replications. Data obtained from all stages of the research will be tested by analysis of variance to obtain variance estimators error and differences between treatments. Furthermore, the data in further testing with the smallest real difference on the real level 1% and 5%.
Results and Discussion
The extraction is done using two methods, the use of water and maceration with methanol. The first method uses hot water as the solvent extract, so that the content of phenols, especially tannins in the leaves preparations can be extracted. Such compounds have properties easily soluble in polar or semi-polar solvents. A hot solvent temperature (above 9000C), can increase theExtractibility compounds in the leaves. The second method, preparations soursop, bay and pegaganleaves are extracted by maceration using methanol. Maceration is a method of extraction by immersing the samples using a suitable solvent in a certain period. This simple method can be used to extract natural ingredient components in the sample that are not resistant to heating (thermolabile) so that damage to the components can be avoided.21
The Activity of Alpha-Amylase
Examination determination antiamylase activity is determined using the method used22 is based on the principle of iodine-starch test. The test results on the inhibition of α-amylase from various extracts, are presented in Table 1.
The average value of a single extract lowest IC50, owned by bay leaves, followed by pegagan leaves and leaves of the soursop, whereas in mixed groups, the average value of the lowest IC50 is owned by a mix of bay leaves and pegaganleaves, but its value is still higher than bay leaves extract. This means that the bay leaves extract had the highest inhibitory activity compared with other extracts, both single group, or a mixture. Anova test results to mean IC50 value of the extracts, either single or mixed, showing a significant difference (p <0.05) (Table 1). Post Hoc LSD test results against the 6 groups, showed there was no significant difference between the mean IC50 bay leaves extract groups to pegaganleavesextract. This means that the leaves of bay and pegagan extract have the same ability effectively to inhibit the activity of α-amylase.
Table 1: The test results of anti α-amylase activity of the leaves extracts
| No | Extracts | IC50 | Average | Standard Deviation | P value | ||
| 1 | 2 | 3 | |||||
| 1 | Bay | 0,451 | 0,413 | 0,429 | 0,431 | 0,019 | 0,0001 |
| 2 | Soursop | 2,428 | 2,404 | 2,339 | 2,390 | 0,046 | |
| 3 | Pegagan | 0,448 | 0,438 | 0,411 | 0,432 | 0,019 | |
| 4 | Bay+Soursop | 1,214 | 1,029 | 1,169 | 1,137 | 0,096 | |
| 5 | Soursop+Pegagan | 0,909 | 0,865 | 0,903 | 0,892 | 0,024 | |
| 6 | Bay+Pegagan | 0,638 | 0,679 | 0,580 | 0,632 | 0,050 | |
The result of the inhibition of plant extracts against α-amylase activity is presented in Figure 1. Sa, Si and Pis an extract of bay, soursop and pegaganleaves. Differences letter on the graph showed that there was a significant difference (p <0.05) based on LSD.
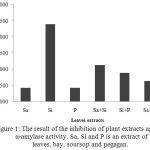 |
Figure 1: The result of the inhibition of plant extracts against α-amylase activity. Sa, Si and Pis an extract of leaves, bay, soursop and pegagan. Differences letter on the graph showed that there was a significant difference (p <0.05) based on LSD.
|
Enzymatic α- amylase inhibitor is a preparation that is used to inhibit the enzymatic activity of α- amylase which plays a role in the digestion of carbohydrates.α-amylase enzyme catalyzes the hydrolysis of starch or polysaccharides into simple sugars/ monosaccharides such as glucose and maltase.
These enzymes are enzymes that hydrolyze protein EC 3.2.1.1 alpha bonds of polysaccharides such as starch and glycogen, into glucose and maltose. This enzyme is the major form found in humans and other mammals. This enzyme is produced by the salivary glands and the exocrine pancreas. Inhibition of α-amylase enzyme activity will affect the process of digestion of carbohydrates and improved blood glucose absorption taking place since chemically treated food in the oral cavity and in the segment of small intestine.
The Activity of Alpha-Glucosidase
Inhibitory activity of various extracts against α-glucosidase enzyme is done using the method of.19 The test results on the inhibition of α-glucosidase from various extracts, are presented in Table 2.
Similar to alpha-amylase, the average value of IC50 extract single room, also owned by the leaves of bay, followed by pegagan leaves and leaves of the soursop, whereas in mixed groups, the average value of IC50 room owned by a mix of bay leaves and leaves of pegagan, but his value is still higher compared bay leaf extract.
The result of the inhibition of plant extracts against α-glucosidase activity is presented in Figure 2. Sa, Si and Pis an extract of leaves, bay, soursop and pegagan. Differences letter on the graph showed that there was a significant difference (p <0.05) based on LSD.
Table 2: The test results of anti α-glucosidase activity of the leaves extracts
| No | Extracts | IC50 | Average | Standard Deviation | P value | ||
| 1 | 2 | 3 | |||||
| 1 | Bay | 0,418 | 0,338 | 0,372 | 0,376 | 0,040 | 0,001 |
| 2 | Soursop | 1,771 | 1,995 | 1,971 | 1,912 | 0,123 | |
| 3 | Pegagan | 0,569 | 0,503 | 0,535 | 0,536 | 0,033 | |
| 4 | Bay+Soursop | 1,082 | 1,094 | 1,035 | 1,070 | 0,031 | |
| 5 | Soursop+Pegagan | 0,922 | 0,881 | 0,850 | 0,884 | 0,036 | |
| 6 | Bay+Pegagan | 0,563 | 0,645 | 0,556 | 0,588 | 0,049 | |
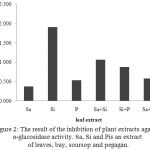 |
Figure 2: The result of the inhibition of plant extracts against α-glucosidase activity. Sa, Si and Pis an extract of leaves, bay, soursop and pegagan. Differences letter on the graph showed that there was a significant difference (p <0.05) based on LSD.
|
This shows that extracts of leaves had the highest inhibitory activity compared with other extracts, either a single group, as well as in the form of a mixture. Anova test results to mean IC50 value of the extracts, either single or mixed, showing a significant difference (p <0.05) (Table 2). Post Hoc LSD test results against the 6 groups, showed that the IC50 value of bay leaves significantly different from the whole extract IC50 another group, whereas among the pegaganleaves, with a mixture of pegaganand bay leavesextract, showed no significant differences. This means, that the bay leavesextract has the ability of most effectiveness to inhibit α-glucosidase activity than other leavesextract, either in single or mixed forms.
Enzymatic α-glucosidase inhibitor is a preparation that is used to lower blood glucose levels by inhibiting the enzyme activity of α-glucosidase. α-glucosidase enzyme is an enzyme produced by the brush border of intestinal villi, which works digest starch or polisaccharida and disaccharide into glucose. Inhibition of this enzymatic activity will affect the absorption and improved blood glucose levels. So that digestion of carbohydrates or starch are extended to reduce the absorption of glucose and increase in blood glucose levels. α-glucosidase enzyme inhibitor is a form of therapy that is effective, safe and tolerable well by diabetics, therefore, the development of herbal medicine will expand and increase the development of therapies in the management of diabetes.
The Content of Total Phenol
Total phenol content is determined using the Folin-Denis reagent.20 The test results showed that the leaves ofthe soursop has a total phenol content of the highest, while pegaganleaves, contains total phenollowest. In mixed groups, a mixture of soursop leaves, and bay leaves,contain the highest total phenol, but not as high as soursop leaves. Full results of the test of total phenol presented in Table 3.
Table 3: Mean of total phenol in extractions
| Extractions | Total Phenol | Mean of total Phenol | Standart Deviation | ||
| 1 | 2 | 3 | |||
| Bay leaf | 3,1 | 3,7 | 2,8 | 3,2 | 0,46 |
| Soursop leaf | 4,8 | 5,4 | 5,1 | 5,1 | 0,30 |
| Pegagan leaf | 2,2 | 2,7 | 1,9 | 2,3 | 0,40 |
| Soursop+ bay | 3,6 | 3,9 | 4,1 | 3,9 | 0,25 |
| Soursop+ Pegagan | 3,2 | 3,7 | 3,3 | 3,4 | 0,26 |
| Bay+ Pegagan | 3,1 | 3,4 | 2,9 | 3,1 | 0,25 |
These test result shows potential total phenol content in extraction and combined extraction, obtained the highest single soursop leavesextract. Potential lowest total phenol content obtained at a single extract of pegagan leaves.
Discussion
Potential total phenol content obtained the highest single soursop leaves extract at 5.1, followed by a mix extract of soursop leavesand bay leaves mix of 3.9 and soursop leavesextract and pegagan leaves of 3.4. Potential total phenol content single bay leavesextract of 3.2 and a mixture of leaves and extracts of pegaganleavesof 3.1. Potential lowest total phenol content obtained at a single extract of pegaganleavesis 2.3.
Soursopleaves, bay leaves, pegaganleaves extract, and the mixture obtained the highest potential anti-α-amylase from a single extract of soursop leaves of 2.39 ± 0.046, followed by a mixture of soursop leaves extract and leaves of 1.137 ± 0.096 and mix extract of soursop leaves and leavesof pegagan of 0.892 ± 0.024. Potential anti-α-amylase extract mixture of bay leaves and pegaganleaves was 0.58 ± 0.05 and the lowest was found in a single extract of pegaganleaves and bay leaves with comparable potency of 0.43 ± 0.019.
Effectiveness of α-glucosidase activity is extraction of herbal and herbal blends found anti α-glucosidase. Potential highest single soursop leaves extract of 1.91 ± 0.123, followed by a mixture of extracts of leaves and leaves of the soursop of 1.07 ± 0.031 and mix extract of soursop and pegaganleaves of 0.88 ± 0.036. Potential anti α-glucosidase mixture extract of pegagan leaves and bay leavesof 0.59 ± 0.049, the single extract of pegaganleaves of 0.57 ± 0.033 and the lowest was found in a single bay leaves extract of 0.43 ± 0.019.
Conclusion
The highest potential effectiveness of anti-α-amylase from a single extract of soursop leaves of 2.39 ± 0.046, followed by a mixture of soursop leavesand bay leavesextract of 1.137 ± 0.096 and mix extract of soursop leaves and pegaganleaves of 0.892 ± 0.024. Potential anti-α-amylase found in the extract lowest single pegagan leaves and bay leaves with comparable potency of 0.43 ± 0.019. The potential effectiveness of anti α-glucosidase single highest soursop leaves extract of 1.91 ± 0.123, followed by a mixture of extracts of bay leaves and leaves of the soursop of 1.07 ± 0.031 and mix extract of soursop and pegaganleaves of 0.88 ± 0.036. Potential anti α-glucosidase obtained at the lowest single bay leaves extracts of 0.43 ± 0.019. Potential total phenolic content obtained the highest single soursop leaves extract at 5.1, followed by a mix extract of soursop leaves and bay leaves mix of 3.9 and soursop leaves extract and pegagan leaves of 3.4. Potential total phenol content single bay leaves extract of 3.2 and a mixture of extracts bay leaves and pegaganleaves of 3.1. Potential lowest total phenol content obtained at a single extract of pegagan leaves of 2.3.
Acknowledgement
This research was supported by the grant of Research Grants and Community Services in Research and Higher Education 2016 Programmed, Skim of “Hibah Bersaing”, University of Lampung, Bandar Lampung, Lampung, Indonesia. Thanks for Ministry of Research and Higher Education of the Republic of Indonesia.
References
- UN Resolution 61/225.http://www.idf.org/diabetesatlas/un-resolution.2009. Accessed January. 2017;25.
- Wild S., Roglic G and Green A. Global Prevalence of Diabetes. Diabetes Care. 2004;27:1047-1053.
CrossRef - Sutanegara D. and The epidemiology and management of diabetesmellitus in Indonesia. 2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgl?.cmd=retrieve. Accessed May. 2017;28.
- Kim S. H and Reaven G. M. Insulin resistance and hyperinsulinemia you can’t have one without the other. Diabetes Care. 2008;31:1433–1438.
CrossRef - Anderson J. Diabetes Mellitus: Medical Nutrition Therapy. In Modern Nutrition in Health and Disease.10th Ed. Shils M. E (Ed.). Lippincott Williams & Wilkins. Philadelpia. 2006;1043-1066.
- Chang C. L. T., Lin Y., Bartolome A. P., Chen Y. C., Chiu S. C and Yang W. Herbal Therapies for Type 2 Diabetes Mellitus: Chemistry, Biology and Potential Application of Selected Plants and Compounds. Evidence-Based Complementary and Alternative Medicine. 2013;33. Article ID 378657.
- Suda T., Takubo K and Semenza G. L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310.
CrossRef - Yen S., Shea M.T., Pagano M, Sanislow C. A., Grilo C.M., Mc.Glashan T. H., Skodol A. E., Bender D. S., Zanarini M. C., Gunderson J. G and Morey L. C. Axis I and Axis II disorders as predictors of prospective suicide attempts: Findings from the Collaborative Longitudinal Personality Disorders Study. Journal of Abnormal Psychology. 2003;112:375–381.
CrossRef - Kati H., Ince I. A., Demir I and Demirbag Z. Brevibacterium pityocampae sp. nov., isolated from caterpillars of Thaumetopoea pityocampa (Lepidoptera, Thaumetopoeidae). Int J Syst Evol Microbiol. 2010;60:312–316.
CrossRef - Barros F. B., Susana A. M. V., Henrique M. P and Luís V. Medicinal use of fauna by a traditional community in the Brazilian Amazonia. Journal of Ethnobiology and Ethnomedicine. 2012;8:37.
CrossRef - Che C. T., Wang Z. J., Chow M. S. S and Lam C. W. Herb-Herb Combination for Therapeutic Enhancement and Advancement: Theory, Practice and Future Perspectives. Molecules. 2013;18:5125-5141.
CrossRef - Thorat L. J., Gaikwad S. M., Nath B. B. Trehalose as an indicator of desiccation stress in Drosophila melanogaster larvae a potential marker of anhydrobiosis. Biochem Biophys Res Commun. 2012;419:638–642.
CrossRef - Giacco and Brownlee. Oxidative Stress and Diabetic Complications. Circulation Research. 2010;107:1058-1070.
CrossRef - Hajizadeh M., Nazmul A and Arijit N. Social inequalities in the utilization of maternal care in Bangladesh: Have they widened or narrowed in recent years?International Journal for Equity in HealthThe official. Journal of theInternational Society for Equity in Health. 2014;13:120.
CrossRef - Quilliot D.., Walters E., Bonte J. P., Fruchart J. C., Duriez P and Ziegler O. Diabetes mellitus worsens antioxidant status in patients with chronic pancreatitis.Am J Clin Nutr. 2005;81(5):1117-25.
CrossRef - Li Y., Zhang W., Zheng D., Zhou Z., Yu W., Zhang L., Feng L., Liang X., Guan W., Zhou J., Chen J., and Lin Z. Genomic Evolution of Saccharomyces cerevisiae under Chinese Rice Wine Fermentation. Genome Biol Evol. 2014;6(9):2516-26.
CrossRef - Hasni M., Azlina A. A and Norhaniza A. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractionsBMC Complementary and Alternative Medicine. International Society for Complementary Medicine Research (ISCMR). 2013;13:118.
- Xiao Z., Storms R and Tsang A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem. 2006;1(1):146-148.
CrossRef - Rao R. R., Tiwari A. K., Reddy P. P., Babu K. S., Ali A. Z., Madhusudana K and Rao J. New furanoflavonoids, intestinal α-glucosidase inhibitory and free radical [DPPH) scavenging, activity from anti hyperglycemic root extract of Derris indica (Lam). Bioorg Med Chem. 2009;17:5170–5175.
CrossRef - Bothon F. T. D., Debiton E., Avlessi F., Forestler C., Teulade J. C and Sohounhloue D. K. In vitro biological effects of two anti-diabetic medicinal plants used in Benin as folk medicine. Complementary and Alternative Medicine. 2013;13:51.
CrossRef - Meloan C. E. Chemical Separations Principles Techniques And Experiments Techniques In Analytical Chemistry” JOHN WILEY & SONS, INC. New York/Chichester/Weinheim/Brisbane/Singapore/Toronto. 1999.
- Xiao Z., Storms R and Tsang A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem. 2006;1(1):146-148.
CrossRef







