Manuscript accepted on :April 15, 2017
Published online on: --
Plagiarism Check: Yes
Rashmi Rajappa1, Salai B. Magesh1, Suryanarayanan Sarvajayakesavulu3 and Subba Rao V. Madhunapantula2
1Division of Water and Health, Faculty of Life Sciences, Jagadguru Sri Shivarathreeshwara University, Mysuru - 570 015, Karnataka, India.
2Center of Excellence in Molecular Biology and Regenerative Medicine (CEMR), Department of Biochemistry, JSS Medical College, Jagadguru Sri Shivarathreeshwara University, Mysuru - 570 015, Karnataka, India.
3SCOPE (Scientific Committee on Problems of the Environment) Beijing Office, P.O. Box 2871, 18 Shuangqing Road, Haidian District, Beijing 100 085, China.
Corresponding Author E-mail: mvsstsubbarao@jssuni.edu.in
DOI : https://dx.doi.org/10.13005/bpj/1145
Abstract
Streptozotocin, a widely used diabetes-inducing agent, is known to cause severe damage to organs that include pancreas, liver and kidneys. This organ damaging effect of streptozotocin is primarily due to its ability to elevate blood glucose, which in turn cause oxidative stress and many metabolic abnormalities. Therefore, antioxidant compounds that can inhibit STZ-induced ROS are potential anti-diabetic agents and help in protecting organs from STZ-mediated damage. Hence, in this study the ability of naringenin, a naturally occurring flavonoid, to protect kidneys from the damage caused by the administration of MLDSTZ in mice was evaluated. Mice treated with intraperitoneal injections of 50mg/kg body weight streptozotocin, for five days, have developed nephropathy, as confirmed by elevated urea, uric acid, creatinine, bilirubin, albumin, and serum thiobarbituric acid reactive substances (TBARS) and lipid hydroperoxides. In addition, a study decline in (a) antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT); (b) glutathione (GSH) and glutathione peroxidase (GPx) and glutathione-S-transferase (GST) was observed in the lysates collected from kidneys. Oral administration of naringenin at 50mg/kg and 100mg/kg body weight for 45 days to STZ treated mice mitigated the complications of nephrotoxicity as demonstrated by normal (or close to normal) levels of renal markers and antioxidants. Furthermore, histopathological examination of kidney sections showed recovery of normal morphology of tissues indicating that naringenin protects mice from STZ-induced kidney damage.
Keywords
Antioxidants Diabetic nephropathy; Naringenin; Streptozotocin;
Download this article as:| Copy the following to cite this article: Rajappa R, Magesh S. B, Sarvajayakesavulu S, Madhunapantula S. R. V. Nephroprotective Effect of Naringenin Against Multiple Low Dose Streptozotocin (MLDSTZ) Induced Renal Damage in Mice. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Rajappa R, Magesh S. B, Sarvajayakesavulu S, Madhunapantula S. R. V. Nephroprotective Effect of Naringenin Against Multiple Low Dose Streptozotocin (MLDSTZ) Induced Renal Damage in Mice. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=14726 |
Introduction
Streptozotocin (STZ) is one of the widely used naturally occurring diabetes-inducing compounds isolated from Streptomyces achromogenes. Chemically it is known as N (Methylnitrosocarbamoyl)-α-D-glucosamine.1 Prior studies have shown that administration of STZ by oral or intravenous routes induces diabetes in mice and rats by destroying pancreatic beta cells.2 In addition to beta cell destruction, STZ is also known to cause damage to other organs such as kidneys (Nephrotoxicity), liver (Hepatotoxicity) and heart (Cardiotoxicity) either through its direct effect or through the complications induced by elevated blood glucose levels.3 Despite these toxic effects, streptozotocin is used as a chemotherapeutic agent for treating pancreatic cancers.4 Therefore strategies to over come the organ toxicity caused by STZ are urgently warranted. Use of natural products, such as flavonoids, is one such strategy to mitigate the complications of STZ-induced damage.5 Flavonoids acts as antioxidants to inhibit reactive oxygen species, which otherwise are detrimental to kidneys and other organs in the body.6 Although various flavonoids such as resveratrol and hesperedin are well explored for their ability to reduce the complications associated with STZ administration, their usage is highly discouraged due to poor bioavailability and limited biological activity.7,8 Hence, an intense search for more bioavailable and effective natural product(s) continued, which identified narigenin as a possible candidate for further testing.
Naringenin is a bioactive flavonoid (Figure 1), widely distributed in citrus fruits.9-11 Prior studies have demonstrated that naringenin could safeguard cells from the damage caused by oxidative stress and inflammatory cytokines.12 Supporting this a study showed the protection from cisplatin induced kidney damage by naringenin treatment.13 Similarly, other studies have reported protection from doxorubicin-induced cardio-toxicity and streptozotocin (STZ) induced hepatic inflammation.14,15 Moreover, recently, naringenin has been reported to be a better agent compared to hesperedin (flavanones) in protecting mitochondrial and cellular membranes.16 Hence, in the present study an attempt was made to evaluate the potency of naringenin to protect kidneys from multiple low-dose streptozotocin induced damage in mice.
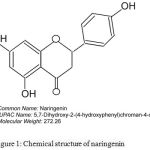 |
Figure 1: Chemical structure of naringenin
|
Materials and Methods
Materials
Naringenin, Streptozotocin, Glibenclamide, and Carboxymethyl Cellulose were procured from Sigma Chemical Company (St Louis, MO). Other chemicals used for this study are of analytical grade.
Animal Experiments
Animal experiments were carried out after receiving approval from the Institutional Animal Ethics Committee (JSSMC/IAEC/18/5675/DEC2013). In brief, male mice weighing about 25-30g were maintained as per the guidelines given by the National Institute of Nutrition, Hyderabad, India and used for the study.
Treatment With Streptozotocin
Mice were injected with STZ (dissolved in 0.1M citrate buffer, pH 4.5) intra-peritoneally at a dose of 50mg/kg/day for 5 consecutive days17 and blood (50 to 100µL) collected from the tail vein. Glucose, in the collected blood, was estimated using Accu-Check Active glucometer to determine whether streptozotocin is functionally active and induced diabetes.18 Mice with blood glucose concentration >250mg/dL were selected for testing the potency of naringenin for mitigating STZ-induced nephrotoxicity as detailed below.19 First, 24 STZ treated and 12 normal mice were divided into a total of 6 groups as shown in Table 1
Table 1: Control and experimental groups of mice:
| Group | Number of mice | Category | Treatment agent | Dose and frequency of the treatment agent (mg/kg body weight) | Route of administration | Comment |
| I | 6 | Normal | Vehicle: Carboxy methyl cellulose | 0.5%, Every day for 45 days | Oral | Normal vehicle control |
| II | 6 | Naringenin* (NAR) | 100mg/kg, every day for 45 days | Oral | Naringenin control | |
| III | 6 | STZ | Streptozotocin (STZ) | 50, 5 consecutive days | Intra peritoneal | STZ control |
| IV | 6 | STZ, followed by NAR* | STZ – 50, 5 consecutive days
NAR – 50mg/kg, every day for 45 days |
Oral | STZ mice treated with low dose naringenin | |
| V | 6 | STZ, followed by NAR* | STZ – 50, 5 consecutive days
NAR – 100mg/kg, every day for 45 days |
Oral | STZ mice treated with high dose naringenin | |
| VI | 6 | STZ, followed by Glibenclamide* | STZ – 50, 5 consecutive days
GLC – 600µg/kg, every day for 45 days |
Oral | A positive control group |
*Naringenin (NAR) and glibenclamide (GLC) were suspended in 0.5% carboxymethyl cellulose.20 At the end of experiment (after 24h of last naringenin administration), the animals were deprived of food overnight and the blood was collected by cardiac puncture.21 Serum was separated, and stored at -20οC until analysis. Kidney tissues, from each animal, were minced and homogenized in 100mM Tris-HCl buffer, pH 7.4, and centrifuged. The supernatant was used for estimation of TBARS, lipid hydroperoxides, SOD, CAT, GSH, GPX and GST.22
Estimation of Biochemical Constituents in the Serum
Concentration of biochemical constituents, whose levels in serum go high when there is a damage to kidneys, such as urea, uric acid, creatinine, albumin and bilirubin was estimated using commercially available kits from Coral Clinical System, Goa, India.
Measurement of STZ-Induced Oxidative Stress
The levels of Lipid peroxidation, in terms of the levels of TBARS formed, was measured using Niehaus and Samuelsson, 1968 method23 and hydroperoxides was measured by the method of Jiang et al., (1992).24 Further, antioxidant enzymes (a) Superoxide dismutase by Kakkar et al., (1984)25; (b) Catalase by Sinha et al., (1972)26; (c) Glutathione peroxidase by Rotruck et al., (1973)27; and Glutathione S-Transferase by Habig et al., (1974)28; and antioxidant molecules such as reduced Glutathione by Ellman’s et al., (1959)29 levels were measured in the renal tissues. For detailed experimental procedures, the readers are suggested to refer the cited references.
Histological Observations of Kidney
At the end of the experiment, kidney tissues were fixed in 10% buffered formalin for 24h30 and dehydrated using ethanol and xylene for 30min each. Tissues were then incubated in liquid paraffin (58ºC) twice for 60min each. Tissue blocks were sectioned into 5μm thickness and stained using hematoxylin-eosin.30 The morphological changes were observed under the light microscope.
Statistical Analysis
All the obtained results were expressed as mean ± SEM of six separate experiments (n = 6). The statistical significance was performed using one-way analysis of variance (ANOVA), SPSS statistics 20 (SPSS, Cary, NC, USA) followed by the Tukey’s post hoc test. P<0.05 was considered as significant.
Results
Oral Administration of Naringenin Reduces the Streptozotocin-Induced Renal Damage
It is well established that damage to kidneys by streptozotocin administration elevates serum urea, uric acid, creatinine, albumin and bilirubin.31 Therefore, to test whether naringenin administration reduces these elevated serum biochemical constituents, first, mice were treated with STZ and damage to kidneys confirmed (Table 1). Next, these mice were treated with 50mg/kg and 100mg/kg body weight naringenin for 45 days, and changes in the biochemical constituents, listed above, measured and compared (before and after naringenin treatment). STZ treatment significantly (P<0.05) increased serum (a) urea from 24.73±0.9 mg/dL to 56.13±4.1 mg/dL; (b) uric acid from 3.81±0.2 mg/dL to 9.38 ±0.2mg/dL; (c) creatinine from 0.69±0.1mg/dL to 1.67±0.3mg/dL; (d) bilirubin from 0.57±0.3mg/dL to 1.28±0.1mg/dL; (e) albumin from 3.39±0.2g/dL to 5.69±0.4g/dL (Table 1). However, administration of naringenin (50 and 100mg/kg b.w.) and positive control compound glibenclamide (600µg/kg b.w.) to STZ-treated mice significantly (P<0.05) decreased the levels of these biochemicals postulating the protective effect of naringenin against STZ-triggered toxicity. For example, the concentration of urea in serum has come down from 56.13±4.1 mg/dL in STZ treated mice to 42.70±2.5 mg/dL and 31.12±1.4 mg/dL, respectively in 50 & 100mg/kg b.w. naringenin treated animals. Similarly other biochemical constituents also showed significant dose dependent reduction upon treatment with naringenin (Table 1)
Oral Administration of Naringenin Retards the Formation of TBARS and Hydroperoxides in STZ-Treated Kidney Tissues
Lipid peroxidation and formation of hydroperoxides are key indicators of tissue damage occurred by oxidative stress.32 STZ is known to induce the levels of lipid peroxides and hydroperoxides in tissues of various organs including kidneys.33 Therefore, first, the levels of lipid peroxides were measured by boiling 0.1ml tissue homogenate with 2.0ml of TBA:TCA:HCl reagent for 15min followed by cooling, and centrifuging at 10,000rpm for 5min to separate clear supernatant. The levels of TBARS (mM / 100g tissue) were estimated by reading the absorption at 535nm. Figure 2A show a significant increase in the concentration of TBARS from about 1mM/100g tissue to ~2.5mM/100g tissue upon STZ treatment. Administration of 50 and 100mg/kg b.w. of naringenin reduced the STZ-induced TBARS to ~1.8mM/100g tissue and ~1.5mM/100g tissue, respectively. The positive control 600µg/kg body weight glibenclimide brought down the TBARS to levels that are similar to control animals (Figure 2A). Treatment of control animals (exposed to vehicle 0.5% carboxymethyl cellulose) with 100mg/kg body weight naringenin did not change TBARS levels (Figure 2A).
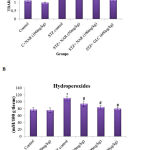 |
Figure 2: Effect of naringenin on the levels of TBARS and hydroperoxides in kidney tissues of control and experimental mice:
|
Increased lipid peroxidation can be due to increased oxidative stress in the cell as a result of impaired activities of antioxidant defense enzymes. The STZ control animals showed a significant (p<0.05) increase in the levels of TBARS and hydroperoxides when compared with the control group (Figure 2A, 2B). Oral administration of naringenin (50 and 100mg/kg b.w.) and glibenclamide (600µg/kg b.w.) showed significantly decreased TBARS and hydroperoxides to near normal levels in kidney tissues of STZ mice (Figure 2A, 2B). Data are expressed as mean ± SE from six mice in each group. Statistical analysis was performed by one-way ANOVA, followed by Turkey post hoc test. *STZ control mice were compared with control mice. #STZ+NAR (50 and 100mg/kg b.w.) and STZ+GLC (600µg/kg b.w.) treated mice were compared with STZ control mice; P < 0.05.
To estimate the concentration of hydroperoxides 0.2ml of tissue homogenate was incubated with 1.8ml of Fox reagent at room temperature for 30minutes and the absorbance measured at 560nm. The results were expressed as mM hydroperoxides produced per 100g tissues. The bar graph in Figure 2B demonstrate that STZ-treatment significantly elevated the tissue hydroperoxides from 76mM/100g tissue to ~116mM/100 tissue. Elevated hydroperoxides were brought down to about 95mM/100g tissue and 84mM/100g tissue, respectively upon 50 and 100mg/kg b.w. naringenin administration (Figure 2B). Oral administration of glibenclamide (600µg/kg b.w.) decreased lipid peroxides to levels that are similar to control animals (Figure 2B). Treatment of control animals (exposed to vehicle 0.5% carboxymethyl cellulose) with 100mg/kg body weight naringenin did not change hydroperoxides levels (Figure 2B).
Oral Administration of Naringenin Enhanced the Activities of SOD, CAT, GSH, GPX and GST in STZ-Treated Kidney Tissues
Increased oxidative stress in tissues modulates the expression of antioxidant defense mechanisms that include enzymes (SOD, CAT, GPX and GST) and other biomolecules (Glutathione). Therefore, the activities of major antioxidant enzymes were measured in control and treated mice. SOD catalyses the dis-mutation of superoxide radicals to H2O2 thereby reducing the possibility of superoxide anion interacting with nitric oxide to form reactive peroxynitrite.34 The SOD activity in the tissues was assayed according to the method of Kakkar et al., (1984).25 The SOD activities were significantly (p<0.05) decreased in the kidney tissue of STZ control animals (from 11.70±0.1 to 6.2±0.1) (Figure 3A). Oral administration of naringenin (50mg/kg and 100mg/kg body weight) and glibenclamide (600µg/kg b.w.) significantly (p<0.05) augmented the levels to near control levels (Figure 3A). Treatment of control animals (exposed to vehicle 0.5% carboxymethyl cellulose) with 100mg/kg body weight naringenin did not change SOD levels (Figure 3A).
Catalase is a tetrameric hemin-enzyme, which decomposes hydrogen peroxide into H2O and oxygen.35 The catalase activity (µmoles of H2O2 consumed/min/mg protein) in the tissues was assayed by the method developed by Sinha et al., (1972).26 The CAT activity significantly (p<0.05) decreased from 42 units in control animals to 28 units in STZ-treated animals (Figure 3B). Oral administration of naringenin (50mg/kg and 100mg/kg body weight) and glibenclamide (600µg/kg b.w.) significantly (p<0.05) augmented the levels to near control concentration (Figure 3B). For example, whereas 100mg/kg body weight naringenin increased the catalase activity to ~40units, the administration of 600µg/kg body weight glibenclimide elevated the levels to about 42 units (Figure 3B). No significant changes were observed in mice treated with 100mg/kg body weight naringenin in comparison to control animals (exposed to vehicle 0.5% carboxymethyl cellulose) demonstrating that naringenin administration has no major impact on catalase activity (Figure 3B).
GPX, a selenium-containing peroxidase, it decomposes H2O2 and lipid peroxides by using GSH, and protects the cells from free radicals.36 The activity of glutathione peroxidase was determined by the method of Rotruck et al., (1973).27 The GPX activity (µmoles of GSH oxidized/min/mg protein) was significantly (p<0.05) decreased from ~8.5µmoles in control animals to ~4.0µmoles in STZ-treated animals (Figure 3C). Oral administration of naringenin (50mg/kg and 100mg/kg body weight) and positive control glibenclamide (600µg/kg body weight) significantly (p<0.05) augmented the levels to that of control animals (Figure 3C). For instance, no significant change in GPX level was observed between control animals and the animals administered with 100mg/kg body weight naringenin or 600µg/kg body weight glibenclimide (Figure 3C). Treatment of control animals (exposed to vehicle 0.5% carboxymethyl cellulose) with 100mg/kg body weight naringenin did not change GPX levels (Figure 3C).
GST is a glutathione-dependent cytosolic enzyme, which protects cells from the damage caused by ROS.37 In order to determine whether administration of naringenin restore the GST activity, a method developed by Habig et al., (1974) was used and the results expressed as µmoles of CDNB-GSH conjugate formed /min/mg protein.28 Analysis of the data represented in Figure 3D showed a 50% decrease in GST activity, compared to vehicle treated mice, when animals were treated with STZ (Figure 3D). Oral administration of naringenin (50mg/kg and 100mg/kg body weight) and glibenclamide (600µg/kg b.w.) significantly (p<0.05) increased the GST activity levels. For example, administration of 100mg/kg body weight naringenin and 600µg/kg body weight glibenclimide elevated the GST levels (from ~2.9 units in STZ treated mice to ~5.6 units in glibenclimide treated mice) that are much closer to the levels observed in control animals (Figure 3D). No significant change in GST activity was observed between vehicle control and 100mg/kg body weight naringenin treated mice (Figure 3D).
GSH is potent free-radical scavenger, a co-substrate for GPX activity, and a cofactor for many redox enzymes.38 Several studies have shown the ability of STZ to reduce cellular glutathione levels. Therefore, to determine whether administration of naringenin could mitigate the effect of streptozotocin-induced GSH depletion effect, kidney tissue was collected from control and experimental animals and processed to estimate glutathione content using Ellman’s assay (Ellman’s et al., (1959).29 The data in Figure 3E showed a significant decrease in tissue GSH levels upon treating mice with STZ (Figure 3E). However, when naringenin or glibenclimide was administered the levels had gone up to the ones present in control animals (Figure 3E). However, treatment of control animals (exposed to vehicle 0.5% carboxymethyl cellulose) with 100mg/kg body weight naringenin did not change GSH levels (Figure 3E).
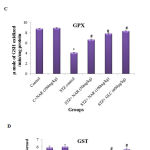 |
Figure 3: Effect of naringenin on the activities of SOD, CAT, GPX, GTS and GSH in the kidney tissues of control and experimental mice:
|
Persistent hyperglycemia leads to increased production of free radicals. STZ control mice showed a significant (p<0.05) decrease in the levels of antioxidants when compared with the control group (Figure 3 A-E). Oral administration of naringenin (50 and 100mg/kg b.w.) and glibenclamide (600µg/kg b.w.) showed significantly increased antioxidants to near normal levels in kidney tissues of STZ mice (Figure 3 A-E). Data are expressed as mean ± SE from six mice in each group. Statistical analysis was performed by one-way ANOVA, followed by Turkey post hoc test. *STZ control mice were compared with control mice. #STZ+NAR (50 and 100mg/kg b.w.) and STZ+GLC (600µg/kg b.w.) treated mice were compared with STZ control mice; P < 0.05.
Oral Administration of Naringenin Restored the Altered Morphology of Kidney Tissues Observed in Mice Treated With STZ
Since STZ administration is known to damage kidneys (nephrotoxicity) by reducing the size and number of functionally active glomerulii, an attempt was made to check whether naringenin and the control glibenclimide restores these altered morphological features.39 The data showed a well-organized renal parenchyma with normal glomeruli and tubules in control animals (Figure 4). However, kidney sections of STZ treated mice exhibited characteristic features of nephrotoxicity such as dilation of renal tubules, necrosis, bowman’s space expansion and swelling of the epithelial cells (Figure 4). These changes in kidney tissue sections were mitigated by the administration of naringenin (Figure 4). While 50mg/kg body weight naringenin showed mild distortion of the histo-architecture of the kidney with moderate necrosis, the ones received 100mg/kg body weight naringenin showed almost normal histo-architecture of the kidney with absence of fibrosis, slight congestion of glomerular tufts, and normal glomeruli (Figure 4). Kidney section of STZ mice treated with positive control compound glibenclamide showed normal histo-architecture of the kidney (Figure 4).
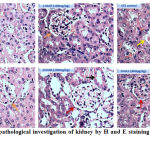 |
Figure 4: Histopathological investigation of kidney by H and E staining (Magnification: 40X)
|
Figure 4 represents the H & E staining of kidney tissues of control and experimental mice. Control and C+NAR (100mg/kg b.w.)- Shows renal parenchyma with normal glomeruli (orange arrow) and tubules; STZ control- necrosis, bowman’s space expansion (yellow arrow) and swelling of the epithelial cells; STZ+NAR (50mg/kg b.w.)- Moderate necrosis (orange arrow). STZ+NAR (100mg/kg b.w.)- Absence of fibrosis, slight congestion of glomerular tufts, and normal glomeruli (brown arrow). STZ+GLC (600μg/kg b.w.)- Normal histoarchitecture of the kidney (brown arrow).
Discussion
Accumulating data suggests that ROS is one of the key contributors for the pathogenesis of STZ induced nephropathy.40 Mechanistically, STZ elevates blood glucose thereby generate excessive ROS through the incomplete oxidation of glucose.2 Unusually high ROS thus generated leads to serious metabolic syndromes such as diabetes.41 In addition, kidneys become more susceptibility to elevated glucose in blood.42 As a result nephropathy occurs in streptozotocin treated mice.42 More over, high blood glucose and excess ROS can suppresses the levels of antioxidant defense enzymes SOD, Catalase, GST and metabolites, which leads to antioxidant imbalance.43 Therefore, restoring normal antioxidant status is very critical to overcome the STZ-induced nephrotoxicity.44 Administration of naturally occurring antioxidant compounds is one way to mitigate the complications of STZ-mediated nephrotoxicity.45 Naringenin is a naturally occurring flavonoid, known to exhibit potent antioxidant properties. Therefore, the potential of naringenin to protect kidney tissues from STZ treatment mediated damage is investigated in this study.
As reported, MLD-STZ triggered renal damage as indicated by increase in serum urea, uric acid, creatinine, bilirubin, albumin and renal tubular changes.46 In addition, STZ reduced the levels of SOD, Catalase, GST and GPX in the kidney tissues.46 In this study we have demonstrated that treatment with 50mg/kg body weight or 100mg/kg body weight naringenin provided marked functional and histological protection against MLDSTZ induced diabetic nephropathy in mice. More over, administration of naringenin decreased the levels of TBARS and hydroperoxides in kidney tissues, implicating reduction in oxidative stress.47 Therefore, naringenin could be a potential candidate for developing a naturally occurring treatment agent for protecting kidneys from oxidative damage. However, further studies are required to elucidate key mechanism(s) by which naringenin exerts its protection.
Conclusion
In conclusion, the current study revealed that naringenin helps to mitigate the STZ-induced complications occurred in kidneys by promoting antioxidant defense enzymes as well as by increasing the levels of glutathione. Hence, naringenin could be used possibly as a potent anti-diabetic agent, but this require additional studies measuring and comparing its’ efficacy with known anti-diabetic agents
Acknowledgements
The authors, Rashmi Rajappa and Salai B. Magesh gratefully acknowledge Jagadguru Sri Shivarathreeshwara University (JSSU), Mysuru for the award of JSS University Research Fellowship.
Conflict of Interest
The authors declare no conflict of interest.
References
- Eleazu C. O., Eleazu K. C., Chukwuma S., Essien U. N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. Journal of Diabetes & Metabolic Disorders. 2013;12:60.
CrossRef - Furman B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015;70:5.47.1-5.47.20.
- Zafar M., Naqvi S. N. H. Effects of STZ-Induced Diabetes on the Relative Weights of Kidney, Liver and Pancreas in Albino Rats: A Comparative Study. Int. J. Morphol. 2010;28:135-142.
CrossRef - Antonodimitrakis C. P., Sundin A., Wassberg C., Granberg D., Skogseid B., Eriksson B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology. 2016;103:345-353.
CrossRef - Yeha W. J., Hsia S. M., Lee W. H., Chi-Hao W. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. Journal of Food and Drug Analysis. 2017;25: 84-92.
CrossRef - Brunetti C., Ferdinando M. D., Fini A., Pollastri S., Tattini M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013;14: 3540-3555.
CrossRef - Kitada M., Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev. 2013;568093.
CrossRef - Pari L., Karthikeyan A., Karthika P., Rathinam A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicology Reports. 2015;2:46-55.
CrossRef - Harris J. W. Solvent extraction of citrus derived products such as citrus juice, oil, concentrate, peel etc. with low molecular weight alcohols and water to reduce concentration of phototoxic furocoumarins. Merck Index.12 ed. Whitehouse Station, NJ: Merck & Co., Inc. 1996.
- Holden J. M., Bhagwat S. A., Patterso K. Y. Development of a multi-nutrient data quality evalution system. Journal of food composition and analysis. 2002;15:339-348.
CrossRef - Sumathi R., Tamizharasi S., Sivakumar T. Bio-dynamic Activity of naringenin-a review. International Journal of Current Advanced Research. 2015;4:234-236.
- Jayaraman J., Jesudoss V. A. S., Menon V. P., Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicology Mechanisms and Methods. 2012;22:568-576.
CrossRef - Badary O. A., Abdel-Maksoud S., Ahmed W. A., Owieda G. H. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci. 2005;76:2125-35.
CrossRef - Subburaman S., Ganesan K., Ramachandran M. Protective role of naringenin against doxorubicin-induced cardiotoxicity in a rat model: histopathology and mRNA expression profile studies. J Environ Pathol Toxicol Oncol. 2014;33:363-76.
CrossRef - Annadurai T., Muralidharan A. R., Joseph T., Hsu M. J., Thomas P. A., Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. Journal of Physiology and Biochemistry. 2012; 68:307-318.
CrossRef - Kapoor R., Kakkar P. Naringenin accords hepatoprotection from streptozotocin induced diabetes in vivo by modulating mitochondrial dysfunction and apoptotic signaling cascade. Toxicology Reports. 2014;1:569-581.
CrossRef - Elango B., Dornadula S., Paulmurugan R., Ramkumar K. M. Pterostilbene ameliorates streptozotocin-induced diabetes through enhancing antioxidant signaling pathways mediated by Nrf2. Chem Res Toxicol. 2016;29:47-57.
CrossRef - Candasamy M., Murthy K., T. E. G., Gubiyappa K. S., Chellappan K., Gupta G. Alteration of glucose lowering effect of glibenclamide on single and multiple treatments with fenofibrate in experimental rats and rabbit models. J Basic Clin Pharm. 2014;5:62–67.
CrossRef - Qinna N. A., Badwan A. A. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Devel Ther. 2015;9: 2515-2525.
CrossRef - Renugadevi J., Milton Prabu S. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Experimental and Toxicologic Pathology. 2010;62:171-181.
CrossRef - Cenedella R. J., Galli C., Paoletti R. Brain free fatty acid levels in rats sacrificed by decapitation versus focused microwave irradiation. Lipids. 1975;10:290-293.
CrossRef - John M. Graham. Homogenization of Mammalian Tissues. The Scientific World Journal. 2002;2: 1626-1629.
CrossRef - Niehaus W. G., Samuelson B. Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126-130.
CrossRef - Jiang Z. Y., Hunt J. V., Woiff S. P. Detection of lipid peroxides using the Fox reagent. Ann. Biochem. 1992;202:384-389.
CrossRef - Kakkar P., Das B., Viswanathan P. N. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130-132.
- Sinha A. K. Colonimetric assay of catalase. Analytical Biochemistry. 1972;47.
- Rotruck J. T., Pope A. L., Ganther H. E., Swanson A. B., Hafeman D. G., Hoekstra W. G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-90.
CrossRef - Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130-7139.
- Ellman G. L., Fiches F. T. Quantitative determination of peptides by sulfhydryl groups Arch. Biochem Biophys. 1959;82:70-72.
CrossRef - Feldman A. T., Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014;1180:31-43.
CrossRef - Badole L. S.,Bodhankar L. S. Antidiabetic activity of cycloart-23-ene-3β, 25-diol (B2) isolated from Pongamia pinnata (L. Pierre) in streptozotocin–nicotinamide induced diabetic mice. European Journal of Pharmacology. 2010;632:103-109.
CrossRef - El-Aal A. M.,H. A. H. Lipid Peroxidation End-Products as a Key of Oxidative Stress: Effect of Antioxidant on Their Production and Transfer of Free Radicals. Biochemistry, Genetics and Molecular Biology. 2012. under CC BY 3.0 license.
- Kinalski M., Sledziewski A., Telejko B., Zarzycki W., Kinalska I. Lipid peroxidation and scavenging enzyme activity in streptozotocin-induced diabetes. Acta Diabetologica. 2000;37:179-183.
CrossRef - Fukai T. T., Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid Redox Signal. 2011;15:1583-1606.
CrossRef - Kirkman H. N., Gaetani G. F. Catalase: A tetrameric enzyme with four tightly boundmolecules of NADPH. Proc. Natl. Acad. Sci. 1984;81:4343-4347.
CrossRef - Lubos E., Loscalzo J., Handy D. E. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957-1997.
CrossRef - Hayes J. D., McLELLAN L. I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radical Research. 1999;31.
- Sies H. Glutathione and its role in cellular functions. Free Radical Biology and Medicine. 1999;27: 916-921.
CrossRef - Zafar M., Naeem-ul-Hassan S. N., Ahmed M., Kaimkhani Z. A. Altered Kidney Morphology and Enzymes in Streptozotocin Induced Diabetic Rats. Int. J. Morphol. 2009;27:783-790.
- Forbes J. M., Coughlan M. T., Cooper M. E. Oxidative Stress as a Major Culprit in Kidney Disease in Diabetes. Diabetes. 2008;57:1446-1454.
CrossRef - Kanwar Y. S., Sun L., Xie P., Liu F., Chen S. A Glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395-423.
CrossRef - Reidy K., Kang H. M., Hostetter T., Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333-2340.
CrossRef - Annadurai T., Muralidharan A. R., Joseph T., Hsu M. J., Thomas P. A., Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68:307-18.
CrossRef - Alsaif M. A. Beneficial Effects of Rutin and Vitamin C Co-administration in a Streptozotocin-Induced Diabetes Rat Model of Kidney Nephrotoxicity. Pakistan Journal of Nutrition. 2009;8: 745-754.
CrossRef - Oyenihi A. B., Brooks N. L.,Oguntibeju O. O., Aboua G. Antioxidant -Rich Natural Products and Diabetes Mellitus, Antioxidant-Antidiabetic Agents and Human Health, Prof. Oluwafemi Oguntibeju (Ed.), InTech, 2014.
- Dabla, P.K. Renal function in diabetic nephropathy. World J Diabetes. 2010;1:48-56.
CrossRef - Prabu S. M., Shagirtha K., Renugadevi J. Reno-protective effect of Naringenin in combination with vitamins C and E on cadmium induced oxidative nephrotoxicity in rats. Journal of Pharmacy Research. 2011;4:1921-1926.







