Manuscript accepted on :May 10, 2017
Published online on: --
Plagiarism Check: Yes
Muhartono1, Asep Sukohar1, Sutyarso2 and Mohammad Kanedi2
1Department of Anatomical Pathology, Faculty of Medicine, University of Lampung, Indonesia.
2Department of Biology, Faculty of Mathematics and Sciences, University of Lampung, Indonesia.
Corresponding Author E-mail: wegayendi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1151
Abstract
Though mucoxin has manufactured and widely marketed online, but indepth study of the role of these annonaceous substances on cellular level is still limited.This study aims to find out more about mucoxin anti-cancer effects, especially those associated with the expression of pro-apoptotic proteins, Bax and p53 in T47D breast cancer cell line.The T47D cell lines was divided into four groupsbased on the hour of mucoxin exposure until assaysdone, namely 0, 24, 48, and 72 hours.Concentration levels of mucoxin applied in theexperiment were 0ng/ml, 0.1ng/ml, 0.5ng/ml, 1ng/ml, 5ng/ml and 10ng/ml. The bax gene expression assayed using quantitative PCR (qPCR) methods, whereas protein expression of bax and p53 determined by immunocytochemistry techniques. The results showed that in comparison with the control, both bax and p53 proteins increase by mucoxin treatment.It is suggested that mucoxin worthy classified as a promising anti-cancer drug.
Keywords
Mucoxin; acetogenin; p53; bax; pro-apoptosis protein; T47D cells; protein expression
Download this article as:| Copy the following to cite this article: Muhartono M, Sukohar A, Sutyarso S, Kanedi M. Mucoxin (Acetogenin) Induce Expression of Pro-Apoptosis Proteins, Bax and P53, in T47D Breast Cancer Cells. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Muhartono M, Sukohar A, Sutyarso S, Kanedi M. Mucoxin (Acetogenin) Induce Expression of Pro-Apoptosis Proteins, Bax and P53, in T47D Breast Cancer Cells. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15158 |
Introduction
Acetogenin is a class of compounds derived from Annonaceae plant family which recently intensively proposed as anti-cancer substances1-3.Among various annonaceous acetogenin derivatives, mucoxin is the latest and claimed the most powerful to eradicate cancer cells because the only type acetogenin contaning a hydroxylated trisubstituted tetrahydrofuran (THF) ring. Mucoxin isa highly potent and specific antitumor agent against number of human cancer cell lines such as A549 lung cancer4, MCF-7 (breast carcinoma) cell lines (ED50 = 3.7 x10(-3)μg/mL compared to adriamycin, ED50 = 1.0 x10(-2)μg/mL)5, and epidermoid carcinoma, K562 and HL-606.
Though mucoxin claimed as a promising anti-cancer, has manufactured and marketed on line all over the world, indepth study of the role of the bioactive materials on cellular level is still limited.In an effort to deepen understanding on the biological activity of mucoxin, Muhartono and colleagues from Indonesia has conducted a series of studies using a T47D breast cancer cell line. Among the results are as follows.Mucoxin effective in suppressing proliferation, increases apoptosis, suppress the expression of cyclin-D1 gene, as well as increasing the expression of the p53 gene in T47D breast cancer cells7,8.
This work is another part of a research project conducted by Murtono and colleagues as an attempt to find out more about the effect of mucoxin treatment on the expression of proliperation and apoptosis related proteins, p53 and bax, in T47D cells.
Methods
The Mucoxin and Cell Lines
Bioactive substances tested in this study was mucoxin (acetogenin) ID AG E 32919 and CASNo. 183195995 obtained from Angene InternationalLimited. The product package contains 5mg of puremucoxin in powder form. Whereas human breastcancer cell lines used in this study was T47D (ATCC®HTB133™) obtained from American Type CultureCollection (Manassas, VA 20108 USA) with a lotnumber 61062006.
Experimental Design
For each protein assessment (p53 and Bax), a randomized block design with six concentrations of treatment and three replications was applied in this study.The T47D cell lines was divided into four groupsbased on the hour of mucoxin exposure until assaysdone, namely 0, 24, 48, and 72 hours.Concentration levels of mucoxin applied in theexperiment are as follows: 0ng/ml, 0.1ng/ml, 0.5ng/ml, 1ng/ml, 5ng/ml and 10ng/ml.
Cell Culture
The cells were grown in Roswell ParkMemorial Institute medium (RPMI 1640) culturemedia supplemented with 10% Foetal BovineSerum (FBS) Gibco™ (from Thermo Fisher ScientificCat. No. 26140 079) and 0.2 units/ml bovine insulin(from Sigma Aldrich Cat. No. I5500 and CAS RN11070 73 8) at 37°C in 5% CO2. Thawing processperformed in waterbath at 37°C for 2-4 minutes.Then, as much as 5×104 cells/cm2 was taken into Tflask and incubated at 37°C in 5% CO2. When cellsdensity reached 80% confluent, trypsinization done using 0.25% Trypsin + 0.53 mM EDTA solution and then subcultured into new culture vessels, also at 37°C in CO2 5%. After two times passaging theT47D cells ready to be treated.
Mucoxin Treatments
Culture media with a volume of 0.5 mL containing approximately 5,000 cells were added to a 24-well culture plate containing gelatin-coated coverslips and incubated for 24 hours. Once the cells density reach 80% confluent the culture media from each well were removed and then serum-free medium and mucoxin of different concentrations in accordance with the treatment levels were added.The cells were then incubated in accordance with the length of hours that have been assigned to each group.
Gene Expression Assay
Because this study is a continuation of research that has been published , in which the gene expression of p53 and cyclin-D1 has been reported, then this section only explains the assessment procedure of the bax gene expression alone.Primers used for determining expression bax gene, refers to Porichi et al.9, was forward primer 5’‒GGACGAACTGGACAGTAACATGG‒3’, and reverse primer5’‒GCAAAGTAGAAAAGGGCGACAAC‒3’. As a control, β-actin gene with the forward primer 5′-TCTGGCACCACACCTTCTACAATG-3′ and reverse primer 5′-AGCACAGCCTGGATAGCAACG-3′ was used.
RNA was extracted from the T47D cell samples using easy-spin™ (DNA free) total RNA extraction kit from Intron Biotechnology. The concentration of total isolated RNA then assessed by NanoDrop 2000 Spectrophotometer from Thermo Scientific (Thermo Fisher Scientific Inc., MA,USA). Expression of bax genes in the T47D cells were determined by quantitative PCR (qPCR) methods using RealMOD™ Green Real-time PCR Kit from Intron Biotechnology with the Cat. No. REF25109. The qPCR data, finally, analyzed using Light Cycler® software from ROCHE. Beta actin (β-actin) gene was used as the internal control (housekeeping gene) while control samples was used as the calibrator genes.
Immunocytochemistry Assays
At the end of the incubation period, the cells were washed using PBS, then fixed in 4% paraformadehid. After being rinsed once with dH2O, the fixed cells washed twice with PBS and then incubated for 5 minutes. Into each well was added 3% H2O2 in PBS at pH 7,4. After being whased three times with PBS and incubated for 5 minutes, blocking-buffer containing 0,25% Triton X‒100 and 5% FBS was added and incubated at room temperature for one hour.After three time wash with PBS and 5 minutes incubation, into each well was added primary antibody with the dilution of 1:100 in PBS with 1% FBS. The primary antibody used for assaying p53 and Bax protein consecutively are p53 protein (wt-p53) polyclonal antibody (Rabbit IgG anti‒p53) from Bioss USA with the Cat. Num. bs‒0033R and anti-Bax polyclonal antibody(Mouse IgG anti‒Bax) from Bioss USA with the Cat. Num. bs‒0127M.
After overnight incubation at 4ºC, the cells then whased three times with PBS and incubated for 5 minutes. Into each well was added the secondary antibody, UltraTek HRP Anti-Polyvalent (DAB) from SkyTex Laboratories with the Cat. Num. AMF080‒IFU incubated at room tempereature for 2 hours. After horseradish peroxidase (HRP) was added the cells were incubated for 40 minutes. After being washed with PBS three times, into the preparates was added 3,3′-diaminobenzidine (DAB), incubated for 40 minutes, and washed with dH2O.
Finally, cells were counterstained with Meyer’s hematoxyline (Dako Cytomation, Denmark) and then washed with dH2O. Coverslips removed from the wells and then mounted on the deck glass coated with 5% gelatin. The resultswere visualized using light microscope Nikon E‒100 and photographed using Nikon Coolpix 4500.To quantify protein expression of p53 and bax, software Image-Pro Plus (IPP) was used.The quantity of protein expression in each treatment was calculated by summing the number of brownnish product arising from the reaction between HRP and DAB in five fields of view and then divided by the number of replicates (n = 3).
Statistical Analysis
Comparison of mean values between treatment are presented as mean ± SDand analyzed using ANOVA followed LSD test.A p-value of less than 0.05 was considered to be statistically significant for ANOVA and α<0.05 was considered to be statistically significant for LSD test.
Results
Effects of Mucoxin on Gene Expression of Bax
Effects of mucoxin treatment on bax gene expression in T47D cells exposed to mucoxin with different concentration at hour 0, 24, 48, and 72are presented in Table 1. Compared to control (0 ng/ml), though not in a consistence manner, mucoxin treatment (0.1 ng/ml – 10 ng/ml) effect on expression of bax gene in all hour groups, even in cell group that were exposed to mucoxin with the duration of less than one hour (0 h).
Tabel 1: Expression of Baxgene (number of copies) in T47D cancer cells treated with mucoxin with differentconcentrations at different exposure times
| Exposure Time | Concentration | Expression | Anova |
| of Mucoxin | (mean±SD) | (P value) | |
| 0 h | 0 ng/ml | 15.857±1.163a | 0.007 |
| 0.1 ng/ml | 10.923±0.351a | ||
| 0.5 ng/ml | 26.967±2.957ab | ||
| 1 ng/ml | 32.980±1.716bc | ||
| 5 ng/ml | 32.533±1.137bc | ||
| 10 ng/ml | 35.233±1.518c | ||
| 24 h | 0 ng/ml | 27.410±0.810ab | 0.011 |
| 0.1 ng/ml | 18.500±3.143a | ||
| 0.5 ng/ml | 35.100±2.078b | ||
| 1 ng/ml | 38.047±1.570c | ||
| 5 ng/ml | 33.334±0.808b | ||
| 10 ng/ml | 36.813±2.161bc | ||
| 48 h | 0 ng/ml | 38.383±0.616a | 0.0001 |
| 0.1 ng/ml | 39.427±1.116ab | ||
| 0.5 ng/ml | 33.550±1.496a | ||
| 1 ng/ml | 52.477±1.390b | ||
| 5 ng/ml | 66.667±1.896c | ||
| 10 ng/ml | 47.500±1.212b | ||
| 72 h | 0 ng/ml | 39.580±1.076a | 0.012 |
| 0.1 ng/ml | 38.747±2.604a | ||
| 0.5 ng/ml | 29.840±1.465a | ||
| 1 ng/ml | 31.453±1.439a | ||
| 5 ng/ml | 31.077±0.901a | ||
| 10 ng/ml | 53.007±2.650b |
Mean±SD values in the same hour group followed by the same superscript are not different at α=0.05 by LSD test.
Effects of Mucoxin on Expression of Bax Protein
Visualization of bax protein expression in T47D breast cancer cells exposed to mucoxin with different concentration at hour 0, 24, 48, and 72 was depicted in Fig.1.The results of quantification of the bax protein expression using the IPP software are presented in Table 2.In contrast to its effects on p53, effects of mucoxin treatment on bax protein expression just evident at 48 hours (p = 0.019) and 72 hours(p = 0.0001). In addition, referring to the results of LSD test (α = 0.05), the highest concentration of mucoxin (10 ng/ml) showsa highest expression of bax protein.
Tabel 2: Expression level of Bax protein in T47Dbreast cancer cellstreated with mucoxin with different concentrations at different exposure times
| Exposure Time | Concentration | Expression | Anova |
| of Mucoxin | (mean±SD) | (P value) | |
| 0 h | 0 ng/ml | 1,333±0,577a | 0.,453 |
| 0.1 ng/ml | 2,000±1,000a | ||
| 0.5 ng/ml | 1,333±1,528a | ||
| 1 ng/ml | 2,667±0,577a | ||
| 5 ng/ml | 2,333±1,528a | ||
| 10 ng/ml | 2,667±0,577a | ||
| 24 h | 0 ng/ml | 2,333±2,517a | 0.164 |
| 0.1 ng/ml | 3,667±0,577a | ||
| 0.5 ng/ml | 5,000±1,000a | ||
| 1 ng/ml | 4,333±1,155a | ||
| 5 ng/ml | 4,667±1,155a | ||
| 10 ng/ml | 5,667±1,528a | ||
| 48 h | 0 ng/ml | 2,000±2,000a | 0.019 |
| 0.1 ng/ml | 5,667±0,577b | ||
| 0.5 ng/ml | 6,000±1,000b | ||
| 1 ng/ml | 7,333±0,577bc | ||
| 5 ng/ml | 7,333±1,528bc | ||
| 10 ng/ml | 8.333±0.577c | ||
| 72 h | 0 ng/ml | 2.000±1.000a | 0.0001 |
| 0.1 ng/ml | 15.000±4.583b | ||
| 0.5 ng/ml | 14.333±3.055b | ||
| 1 ng/ml | 29.000±6.557c | ||
| 5 ng/ml | 30.0001±4.359c | ||
| 10 ng/ml | 28.333±2.517c |
Mean±SD values in the same hour group followed by the same superscript are not different at α=0.05 by LSD test.
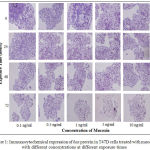 |
Figure 1: Immunocytochemical expression of bax protein in T47D cells treated with mucoxin with different concentrations at different exposure times
|
Effects of Mucoxin on Protein Expression of p53
Immunocytochemicalexpression of p53 protein in T47D breast cancer cells exposed to mucoxin with different concentration at hour 0, 24, 48, and 72 were visualized in Fig.2.The quantification of the p53 protein expression using IPP software resulted in the data presented in Table 3. Referring to the p-value of Anova in the table, it appears that mucoxin already showing effect within 24 hours (p = 0.001), and continues to increase at 48 hours (p = 0.045) and 72 hours (p = 0.009).In all groups of hours, as compared with the control group (0 ng/ml), mucoxin of all concentrations significantly increased the expression of p53 protein(α = 0.05). At the end of hour 72, the effect of mucoxin from the lowest (0.1 ng/ml) to the highest (10 ng/ml) is significantly different, the higher the concentration of mucoxin given the higher expression of p53 protein (α = 0.05).
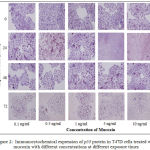 |
Figure 2: Immunocytochemical expression of p53 protein in T47D cells treated with mucoxin with different concentrations at different exposure times
|
Tabel 3: Expression level of p53 protein in T47D breast cancer cellstreated with mucoxin with different concentrations at different exposure times
| Exposure Time | Concentration | Expression | Anova |
| of Mucoxin | (mean±SD) | (P value) | |
| 0 h | 0 ng/ml | 4.333±1.528a | 0.565 |
| 0.1 ng/ml | 4.333±0.577a | ||
| 0.5 ng/ml | 3.667±0.577a | ||
| 1 ng/ml | 5.000±1.000a | ||
| 5 ng/ml | 4.333±0.577a | ||
| 10 ng/ml | 4.333±0.577a | ||
| 24 h | 0 ng/ml | 2.333±2.017a | 0.001 |
| 0.1 ng/ml | 8.333±1.528b | ||
| 0.5 ng/ml | 13.333±3.055bc | ||
| 1 ng/ml | 11.667±3.786bc | ||
| 5 ng/ml | 13.667±3.055bc | ||
| 10 ng/ml | 14.000±2.000c | ||
| 48 h | 0 ng/ml | 3.333±3.055a | 0.045 |
| 0.1 ng/ml | 13.333±4.041b | ||
| 0.5 ng/ml | 15.333±2.082bc | ||
| 1 ng/ml | 14.333±3.215bc | ||
| 5 ng/ml | 17.333±1.528bc | ||
| 10 ng/ml | 18.333±0.577c | ||
| 72 h | 0 ng/ml | 5.000±3.606a | 0.009 |
| 0.1 ng/ml | 18.333±2.082b | ||
| 0.5 ng/ml | 34.333±3.055c | ||
| 1 ng/ml | 79.000±26.514d | ||
| 5 ng/ml | 86.667±27.429d | ||
| 10 ng/ml | 145.000±72.959e |
Mean±SD values in the same hour group followed by the same superscript are not different at α=0.05 by LSD test.
Discussion
As has been previously reported that in the T47D breast cancer cells mucoxin shown to increase p53 gene expression and decreasing gene expression of cyclin-D17, in addition mucoxin also proven to decrease proliferation and increase apoptosis of the cell lines8.In current study it was found that mucoxin treatment induce expression of bax gene (Table 1), promote expression of bax protein (Fig.1 and Table 2) as well as p53 protein (Fig.2 and Table 3).
Increased expression of Bax by mucoxin can be explained by several mechanisms. Mucoxin allegedly can activate, stabilize, and accumulate Bax in the mitochondria to function as an proapotosis agent.Annonacin, is one type of asetogenin, as reported by Yuan et al.10, can cause cell cycle stops at the G1 phase and causes cytotoxic through Bax and caspase pathway.Bax is a proapoptosis protein that promotes cell death upon the presence of stress stimuli, that in this study the stress likely caused by mucoxin.11
In cancer cells, apoptosis induction was correlated with inhibition of bax degradation12.The increase in the degradation of Bax is one way for cancer cells to survive13. Whereas in cancer cells treated with mucoxin, bax will be oligomerized easily in mitochbondrial membranbe that make the protein more stable. All bax molecules become oligomerized after membrane insertion and are present at pore edges. Such oligomerization may lead to membrane deformation that promote pore enlargement14.
Due to pores enlargement, mitochondrial membrane become more permeable and release mitochondrial cytochrome c, Smac andAIF into the cytosol and activation of caspase-mediated apoptosis15.Next, cytochrome c binds to the apoptosis-activating factor 1 (Apaf-1) and procaspase 9 forms apoptosom which will activate caspase pathway leading to apoptosis16.Given mucoxin belonged to the annonaceous acetogenin family, then there is no harm if mucoxin analogized with other acetogenin such as bullatacin.Bullatacin, as indicated by Chen et al.17, is an acetogenin substances that was able to increase of reactive oxygen species (ROS). Accumulation of ROS in cancer cells will activate Bax and caspase pathway which will further initiate apoptosis18.In short, the pro-apoptotic protein Bax commits a cell to death bypermeabilizing the mitochondrial outer membrane(MOM)19.
As has been shown in Fig.2 and Table 3, all concentration of mucoxin treatment significantly increase expression of p53 gene. However there is little study that could explain satisfactorily about mucoxin mechanisms in inhibiting the proliferation and increasing apoptosis whether through increased stabilization and activation of p53.Yuan et al.20 from Taiwan, by testing cytotoxicity effects of annonacin on prostate cancer cells concluded that annonacin can arrest cell cycle at phase S1 depended on caspase 3 and bax. In Indonesia, Rachmawati et al. 21 has also managed to identify that activation and stabilization of p53 play a key role in apoptosis of cervical cancer cells.The activated p53 will act as a regulatory protein that triggers a variety of biological responses mainly in the process of cell proliferation and apoptosis22.
When p53 is active, there will be phosphorylation at one or more serine residues on the N-Terminus or C-Terminus which would then be bound to enhancer elements that are the downstream targets of p5323.The downstream targets of p53 will then respond the binding by self activation or not24. Until now it has known hundreds of p53 target genes with various functions and cellular mechanisms.In general, the p53 target genes grouped in three main pathways as the downstream activation of p53 including cell cycle arrest when DNA damage occurs, repair of damaged DNA, and control apoptosis when the damage can not be repaired anymore25.
In relation to bax protein, Maximov and Maximov26suggested that the p53 tumor-suppressor protein can intervene at every major step in apoptotic pathways. As indicated by Schuler et al.27that p53 activates the apoptotic machinerythrough induction of the release of cytochrome c from the mitochondrial intermembrane space. The release of cytochrome c facillitated, of course , by pore enlargement of the mitochondrial membrane as the bax activity.
Based on the fact that mucoxin significantly increase gene expression and protein expression of Bax as well as p53 protein, wherein both proteins are the main factor in the apoptosis of cancer cells, it can be concluded that mucoxin deserves to be called an anti-cancer substance.
Acknowledgement
Authors are grateful for the supports from Faculty of Medicine, University of Lampung, Indonesia.
Conflict of Interest
Authors declare no conflict of interest.
References
- Wang L.Q., Nakamura N., Meselhy M.R., Hattori M., Zhao W.M., Cheng K.F., et al. Four mono tetrahydrofuran ring acetogenins, montanacins B E, from Annona montana. Chem Pharm Bull 2000; 48(8):1109 1113.
CrossRef - Formagio ASN, Kassuya CAL, Neto FF, Volobuff CRF, Iriguchi EKK, Vieira MC, Foglio MA. The flavonoid content and anti proliferative, hypoglycaemic, anti inflammatory and free radical scavenging activities of Annona dioica St. Hill. J Altern Complement Med 2013; 13(14):1-8.
- Rachmawati E., Karyono S., Suyuti H. The effect of Annona muricata leaf on proliferation and appoptosis of HeLa cells mediated by p53. JKB 2012; 27(1):28 32.
CrossRef - Shi G., Kozlowski J.F., Schwedler J.T., Wood K.V., MacDougal J.M., McLaughlin J.L. Muconin and mucoxin: additional nonclassical bioactive acetogenins from Rollinia mucosa. J Org Chem. 1996; 61: 7988–7989
CrossRef - Narayan R.S., Borhan B. Synthesis of the proposed structure of mucoxin via regio and stereoselective tetrahydrofuran ring forming strategies. J Org Chem 2006; 71(4):1416 1429.
CrossRef - Garzan A.I. Synthesis of ether linked analogs and SAR studies of Annonaceous acetogenins II. Asymmetric electrophilic halocyclization reactions. A D. Dissertation Michigan State University. 2012. pp: 333.
- Muhartono, Sutyarso and Kanedi M.,Mucoxin (Acetogenin) Inhibits Proliferation of T47D Breast Cancer by Suppressing Expression of Cyclin D1 Mediated by p53. Int. J. Cancer Res., 2016; 12(2): 101-108.
CrossRef - Muhartono, Sukohar A., Sutyarso and Kanedi M. Anti-proliferative and Apoptotic Effects of Mucoxin (Acetogenin) in T47D Breast Cancer Cells. & Pharmacol. J., 2016; 9(2), 491-498.
- PorichiO.,Nikolaidou M.E., Apostolaki A., Tserkezoglou A., Arnogiannaki N., Kassanos D. et al., BCL‒2, BAX and p53 expression profiles in endometrial carcinoma as studied by Real‒time PCR and immunohistochemistry. Anticancer Res., 2009; 29:3977‒3982.
- Yuan S.S.F., ChangL., Chen H.W.,Yeh Y.T., Kao Y.H., Lin K.H., Wu Y.C., Su J.H. Annonacin, a mono‒tetrahydrofuranacetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Bax and caspase 3related pathway. Life Sci, 2003;72(25):2853‒2861.
CrossRef - Walensky L.D. and Gavathionis E. Bax unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Bioshem Sci., 2011; 36(12):642–6
CrossRef - Bagci E.Z, Vodovotz Y., Billiar T.R., Ermentrout G.B. and BaharI. Bistability in apoptosis: Roles of Bax, Bcl–2, and mitochondrial permeability transition pores. Biophys J., 2006; 90(5): 1546–1559.
CrossRef - Li B. andDou Q.P.Bax degradation by the ubiquitin/proteasome–dependent pathway: involvement in tumor survival and progression.Proc Natl Acad Sci USA,2000;97(8):3850–385
CrossRef - Kuwana T., Olson N.H., Kiosses W.B., Peters B. and Newmeyer D.D. Pro-apoptotic Bax molecules densely populate the edges of membrane pores. Sci Rep. 2016; 6:27299
CrossRef - Green D.R. and Chipuk J.E. Apoptosis: stabbed in the Bax. Nature, 2008; 455:1047‒
CrossRef - Thomadaki H. and Scorilas A. BCL2 family of apoptosis–related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci., 2006; 43(1):1–67.
CrossRef - [17] Chen Y., Chen J.W., Zhai J.H., Wang Y., Wang S.L., Li X.Antitumor activity and toxicity relationship of annonaceous acetogenins. Food Chem Toxicol,2013; 58:394–400.
CrossRef - Byun J.Y., Kim MJ, Eum D.Y., Yoon C.H., Seo W.D., Park K.H., Hyun J.W., Lee Y.S., Lee J.S., Yoon M.Y., Lee S.J. Reactive oxygen species–dependent activation of Bax and Poly(ADP–ribose) Polymerase‒1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol Pharmacol, 2009; 76(4):734–744.
CrossRef - Brahmbhatt H., Uehling D., Al-awar R., Leber B. and Andrews D. Small molecules reveal an alternative mechanism of Bax activation. Biochem. J., 2016; 473:1073–1083
CrossRef - Yuan SSF, Chang HL, Chen HW, et al., 2006. Selective cytotoxicity of squamocin on T24 bladder cancer cells at the S‒phase via a Bax, Bad, and caspase 3related pathways. Life Sci78:869‒
- Rachmawati E., Karyono S. and Suyuti H. The effect of annona muricata leaf on proliferation and appoptosis of HeLa cellsmediated by p53. JKB, 2012; 27(1):28‒32.
- Mollereau B, Ma D, 2014. The p53 control of apoptosis and proliferation: lessons from Drosophila. Apoptosis 19:1421–1429.
CrossRef - Ou YH, Chung PH, Sun TP, et al., p53 C‒Terminus phosphorylation by CHK1 and CHK2 participates in the regulation of DNA‒damage‒induced C‒Terminus acetylation. Mol Biol Cell 16:1684 –1695.
- Bai L. and Zhu W.G. p53: structure, function and therapeutic applications. J Cancer Mol, 2006); 2:141-53.
- Ozaki T. and Nakagawara A. Role of p53 in Cell Death and Human Cancers. Cancers, 2011; 994-1013.
CrossRef - Maximov G.K. and Maximov K.G. The Role of p53 Tumor-Suppressor Protein in Apoptosis and Cancerogenesis. & Biotechnol. EQ. 22/2008/2:664-668.The p53 tumor-suppressor protein can intervene at every major step in apoptotic pathways.
- Schuler M., Bossy-Wetzel E., Goldstein J.C., Fitzgerald P. and Green D.R. 2000. p53 Induces Apoptosis by Caspase Activation through Mitochondrial Cytochrome c Release. J Biol Chem 275(10): 7337–7342.
CrossRef







