Manuscript accepted on :May 23, 2017
Published online on: --
Plagiarism Check: Yes
Nitya Rudraraju1,2, Animisha Mokkapati1, Radhakrishna Nagumantri1, Chinna Babu Pydi1, Ramakrishna Chintala3 and Satyanarayana Rentala1
1Department of Biotechnology, Institute of Technology, GITAM University, Gandhi Nagar, Visakhapatnam 530 045 Indai.
2School of Engineering, Boston University, Massachusetts USA.
3GITAM Institute of Science, GITAM University, Gandhi Nagar, Visakhapatnam 530 045.
Corresponding Author E-mail: dr.satya@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1155
Abstract
A daily diet rich in spices may offer protection against cancer and other illnesses. This may be reason, why Indians suffer lower cases of many cancers. One of the most widely traded spices in the world, Piperine, the main alkaloid from black pepper has been shown to substantially increase the bioavailability of the nutrients in foods and supplements. Piperic acid is a chemical obtained by the hydrolysis of the alkaloid piperine from black pepper, followed by acidification of the corresponding salt. The cytotoxic effects of piperic acid are hither to unknown. In this paper, the cytotoxic effects of piperic acid in prostate cancer cells (PC-3) and breast cancer cells (MDA-MB-231) were studied. The drug treatment experiments clearly indicated that maximum cytotoxicity was achieved at 48 hours and at 100µM concentration of piperic acid in both the cell lines.
Keywords
Piperic acid; Cytotoxicity; Prostate cancer cell lines; Breast cancer cell lines and Mononuclear cells
Download this article as:| Copy the following to cite this article: Rudraraju N, Mokkapati A, Nagumantri R, Pydi C. B, Chintala R, Rentala S. In Vitro Evaluation of Cytotoxic Properties of Piperic Acid. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Rudraraju N, Mokkapati A, Nagumantri R, Pydi C. B, Chintala R, Rentala S. In Vitro Evaluation of Cytotoxic Properties of Piperic Acid. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15346 |
Introduction
Ayurvedic medicine is a system of diagnosis and treatments that has been practiced in India for more than 2500 years. The term “ayurveda” comes from Sanskrit. It means “knowledge of life”. Ayurvedic theory holds that the human body represents the entire universe in microcosmic form, and that we come to know how we function as organisms only by observing and understanding the world around us. Modern pharmaceutical research is concerned with all aspects of identifying new chemical substances with new modes of action.
The fruit of the Black Pepper plant, Black Pepper is useful both as a spice and an Ayurvedic medicine1. The spiciness in Black Pepper is due to the chemical Piperine. One of the most widely traded spices in the world, Piperine, the main alkaloid from black pepper has been shown to substantially increase the bioavailability of the nutrients in foods and supplements2,3. Piperic acid is a chemical often obtained by the base-hydrolysis of the alkaloid piperine from black pepper, followed by acidification of the corresponding salt.4,5,6,7 Piperic acid is an intermediate in the synthesis of other compounds such as piperonal, and as-such may be used to produce fragrances, perfumes flavorants and drugs as well as other useful compounds8,9,10,11. The structure of piperic acid was given in Figure 1.
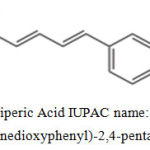 |
Figure 1: Piperic Acid IUPAC name: (2E,4E)-5-(3,4-methylenedioxyphenyl)-2,4-pentadienoic acid
|
The cytotoxic effects of piperine, an alkaloid found in black pepper, have been studied extensively12,13,14,15. However the cytotoxic activity of its derivative, piperic acid has not been properly investigated. The present paper is focused on the research works to fill the gap and examine the cytotoxic effects of piperic acid.
Materials and Methods
Materials used
DMEM (Dulbecco’s Modified Eagle Medium), MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) FBS (Fetal Bovine serum), DMSO (DiMethyl Sulphoxide), 96 well micro plate, Inverted Microscope, Micropipettes, Micro tips, Ficoll Histopaque 1077, Piperic acid procured from Sigma-Aldrich, India, Trypsin-EDTA solution 1X w/ 0.025% Trypsin and 0.01% EDTA in Dulbecco’s Phosphate Buffered Saline Sterile filtered.
Cell Lines used
PC-3 (Prostate cancer) and MDA-MB-231 (Metastatic Breast Cancer) Cell lines was obtained from NCCS (National centre for cell science), Pune, India. The PC-3 and MDA-MB -231 cells were cultured in DMEM supplemented with 10% FBS and antibiotics. The cell lines were maintained at 37°C in a 5% CO2 incubator.
Culturing of Mononuclear Cells from Peripheral Blood
5ml peripheral blood was collected in heparininzed blood collection tubes.
The sample was carefully added to the layer of the Ficoll Histopaque 1077 (Lymphocyte separating medium whose density is 1.077 times higher than peripheral blood) (1:1) in 15ml tubes. The tubes were centrifuged at 7000 rpm for 30 min without brake. The buffy coat was carefully aspirated and the mononuclear dense ring was collected in fresh sterile 15ml centrifuge tubes. The collected cells were washed two times using 10ml DMEM. The cell pellet was suspended DMEM enriched with 10% heat- inactivated fetal bovine serum and antibiotics. The cells were maintained in 5% CO2 incubator at 370C.
Drug Treatment and Cell Viability / Cytotoxicity Analysis using MTT Assay
Mononuclear cells were obtained as mentioned previously and cultured in DMEM serum containing medium. The trypsinised cancer cells from T-25 flask were incubated in a 96 well plate and allowed to adhere to the wells overnight in CO2 incubator. Every time 5000 cells per well were taken. Cell lines were treated with different concentrations of piperic acid (1,10,100 µM solutions). After treatment at various time intervals (24, 48 and 72 hours) , 20µl of MTT (5mg/ml in PBS) was added into each well and incubated in a CO2 Incubator until purple precipitate was visible. Then the supernatant was discarded and 200 µl of DMSO was added to each well to dissolve formazan crystals. The absorbance was read at a wavelength of 492nm on microplate reader
The following formula was used to calculate cell viability
% Cell viability: (Test OD492/ Control OD 492 ) × 100
% Cytotoxicity = 100 – % Cell viability
Results and Discussions
MDA-MB-231, PC-3 and Mononuclear cells were cultured in 96 well plates. Figure 2 shows the cultured MDA-MB-231 (Breast cancer), PC-3(Prostate cancer) and Mononuclear cells.
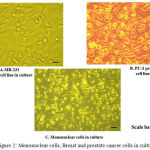 |
Figure 2: Mononuclear cells, Breast and prostate cancer cells in culture
|
To test the cytotoxicity. Prostate cancer cells (PC-3 cell line) and breast cancer cells (MDA-MB-231 cell line) and Mononuclear cells were cultured.
Once the MTT assay was conducted on the MNCs and MDA-MB-231, after the incubation period the cell viability was calculated and the following graph was obtained.
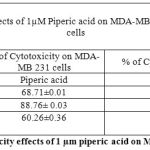 |
Table 1: % of Cytotoxicity effects of 1 µm piperic acid on MDA-MB-231 and MNCs
|
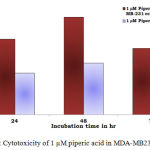 |
Figure 3: Cytotoxicity of 1 µM piperic acid in MDA-MB231 and MNCs
|
After the cells were treated with 1 micromolar concentrations of piperic acid (as shown in Table 1 and Figure 3), it was found that the cell death was much higher in the metastatic breast cancer cell lines than the mononuclear cells. This is certainly ideal as this indicates the drug can probably be safely injested into the body without causing harm to healthy cells. From the graph it was understood that piperic acid interfere with the DNA replication process hence halting the cell cycle and replication. The cytotoxic properities of 10 µM Piperic acid on MDA-MB 231 and mononuclear cells were given in Table 2 and Figure 4.
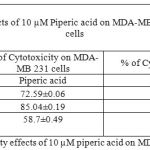 |
Table 2: % of Cytotoxicity effects of 10 µM piperic acid on MDA- MB 231 and MNCs
|
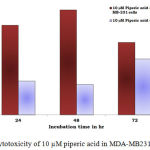 |
Figure 4: Cytotoxicity of 10 µM piperic acid in MDA-MB231 and MNCs
|
The results obtained after treatment of 10 µM piperic acid were given in Table 2 and Figure 4. In both types of cells cytotoxicity appeared to have peaked after 48 hours of incubation. This seemed to be the point where the cells had completely utilized the entire drug that was added. It is important to note that a decrease in cytotoxicity does not indicate that cell death has been reversed in any way, since these values were calculated relative to control. The cytotoxic properities of 100 µM Piperic acid on MDA-MB 231 and mononuclear cells were given in Table 3 and Figure 5.
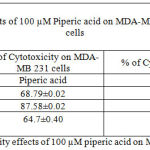 |
Table 3: % of Cytotoxicity effects of 100 µM piperic acid on MDA-MB 231 and MNCs
|
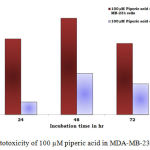 |
Figure 5: Cytotoxicity of 100 µM piperic acid in MDA-MB-231 and MNCs
|
The results obatined at 100 micromolars also exhibits a similar trend to the previous concentrations. The percentage of cytotoxicity was maximum at this particular concentration of piperic acid in the MDA-MB-231 cells.
Once the MTT assay was conducted on the MNCs and PC-3 cells, after the incubation period the cell viability was calculated and the following graph was obtained.
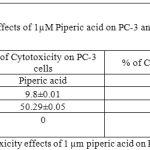 |
Table 4: % of Cytotoxicity effects of 1 µm piperic acid on PC-3 and MNCs
|
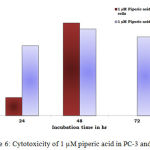 |
Figure 6: Cytotoxicity of 1 µM piperic acid in PC-3 and MNCs
|
After the cells were treated with 1 micromolar concentrations of piperic acid (Table 4 and Figure 6), it was found that the cell death was much higher in the metastatic breast cancer cell lines than the mononuclear cells. This is certainly ideal as this indicates the drug can probably be safely injested into the body without causing harm to healthy cells. From the graph it was understood that piperic acid interfere with the DNA replication process hence halting the cell cycle and replication. The cytotoxic properities of 10 µM Piperic acid on PC-3 and mononuclear cells were given in Table 5 and Figure 7.
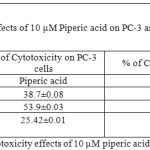 |
Table 5: % of Cytotoxicity effects of 10 µM piperic acid on PC-3 and MNCs
|
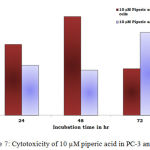 |
Figure 7: Cytotoxicity of 10 µM piperic acid in PC-3 and MNCs
|
The results obtained after treatment of 10 µM piperic acid were given in Table 5 and Figure 7. In both types of cells cytotoxicity appeared to have peaked after 48 hours of incubation. This seemed to be the point where the cells had completely utilized the entire drug that was added. It is important to note that a decrease in cytotoxicity does not indicate that cell death has been reversed in any way, since these values were calculated relative to control. The cytotoxic properities of 100 µM Piperic acid on PC-3 and mononuclear cells were given in Table 6 and Figure 8.
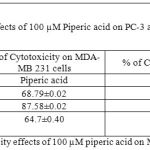 |
Table 6: % of Cytotoxicity effects of 100 µM piperic acid on MDA- MB 231 and MNCs
|
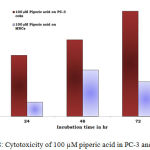 |
Figure 8: Cytotoxicity of 100 µM piperic acid in PC-3 and MNCs
|
The results obatined at 100 µM also exhibits a similar trend to the previous concentrations. The percentage of cytotoxicity was maximum at this particular concentration of piperic acid in the PC-3 cells.
In the prostate cancer (PC-3) cells when treated with piperic acid at different time periods of incubation, it was estimated that the optimal concentration was 100 µM.
Conclusions
It is important to understand the mechanism of cell death in MDA-MB231 and PC-3 cells when treated with piperic acid. Both the cell lines were treated with piperic acid in different concentrations (1,10 and100 micromolars), at different time periods of incubation (24,48,72 hours.) The mononuclear cells were isolated from healthy human volunteers peripheral blood. These MNCs were also treated as mentioned above. From these experiments it was estimated that 100 micromolar concentration was optimal to obtain the maximum cytotoxicity in cancer cells. This concentration did not affect the cell viability of the mononuclear cells. At all the concentrations the optimal time of incubation was measured to be 48 hours. After this time period the cytotoxicity was reduced when measured at 72 hours. Perhaps the cytotoxicity would have been maintained if a second dose of the drug were administered. This decrease in cell death indicates that after the drug had been completely utilized by the cells and shown its effect, the few cells which were still alive after 48 hours of incubation began to multiply thereby decreasing the percentage of cytotoxicity.
Acknowledgements
We are thankful to University Grants Commission, Govt of India for sponsoring the project (file number 42-221/2013 (SR)). We are also thankful to GITAM University for providing necessary infrastructure to conduct the research works communicated in the paper.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Durvasula V.R. Venugopal, Nagendra Sastry Yarla and Parimi Umadevi. Synthesis, of Novel Piperine Analogs of Dipeptidyl Boronic Acid as Antimicrobial and Anticancer Agents. Med chem 2014, 4:9, 606-610
- Lesa D. Fraker, Susan A. Halter, and James T. Forbes. Growth Inhibition by retinol of a Human Breast Carcinoma Cell Line in vitro and in Athymic Mice. Cancer Research 44, 5757-5763, 1984
- Satyendra Mishra, Upma Narain, Roli Mishra and Krishna Misra. Design, development and synthesis of mixed bioconjugates of piperic acid–glycine, curcumin–glycine/alanine and curcumin–glycine–piperic acid and their antibacterial and antifungal properties. Bioorganic & Medicinal Chemistry 13 (2005) 1477–1486
CrossRef - Umadevi P, Deepti K, Venugopal DV. Synthesis, anticancer and antibacterial activities of piperine analogs. Med Chem Res 2013; 22:5466‑71.
CrossRef - Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes.Pharmacol Biochem Behav 2009; 92: 39–43.
CrossRef - Bang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, Kim JY,et al. Anti-inflammatory and anti-arthritic effects of piperine in human interleukin-1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther 2009; 11: R49.
CrossRef - Kumar S, Singhal V, Roshan R, Sharma A, Rembhotkar GW, Ghosh B. Piperine inhibits TNF-alpha induced adhesion of neutrophils to endothelial monolayer through suppression of NF-kappaB and IkappaB kinase activation.Eur J Pharmacol 2007; 575: 177–86.
CrossRef - Wattanathorn J, Chonpathompikunlert P, Muchimapura S, Priprem A, Tankamnerdthai O. Piperine, the potential functional food for mood and cognitive disorders.Food Chem Toxicol 2008; 46: 3106–10.
CrossRef - Taqvi SI, Shah AJ, Gilani AH. Blood pressure lowering and vasomodulator effects of piperine.J Cardiovasc Pharmacol 2008; 52: 452–8.
CrossRef - Pathak N, Khandelwal S. Cytoprotective and immunomodulating properties of piperine on murine splenocytes: anin vitro Eur J Pharmacol 2007; 576: 160–70.
CrossRef - Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects.Crit Rev Food Sci Nutr 2007; 47: 735–48.
CrossRef - Pradeep CR, Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice.Clin Exp Metastasis 2002; 19: 703–8.
CrossRef - Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, Chung YC,et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCalpha/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett 2011; 203: 9–19.
CrossRef - Bezerra DP, de Castro FO, Alves AP, Pessoa C, de Moraes MO, Silveira ER,et al. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J Appl Toxicol 2008; 28: 156–63.
CrossRef - Bezerra DP, Castro FO, Alves AP, Pessoa C, Moraes MO, Silveira ER, et al. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz J Med Biol Res 2006; 39: 801–7.
CrossRef







