Manuscript accepted on : April 17, 2017
Published online on: --
Plagiarism Check: Yes
Sareh Saadat1,2, Jalal Mardaneh3, Mehrdad Ahouran4, Alireza Mohammadzadeh3, Abdollah Ardebili5, Masoud Yousefi6 and Mohammad Mansouri7
1Microbiology Department, Islamic Azad University, Jahrom Branch, Iran.
2Young Researchers club, Jahrom Branch, Islamic Azad University, Jahrom, Iran.
3Department of Microbiology, School of Medicine, Gonabad University of Medical Sciences, Gonabad, Iran.
4Department of pathobiology, Veterinary College, Mashhad University of Medical Sciences, Mashhad, Iran.
5Department of Microbiology, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran.
6Department of Microbiology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
7Quality Control Microbiology Laboratory, Tehranile Pharmaceutical Company, Karaj, Iran.
Corresponding Author E-mail: icft2010@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1181
Abstract
Brucellosis is one of the most widespread zoonotic diseases globally. This chronic, contagious disease mainly transmitted from cattle, sheep, goats, pigs and camels through direct contact with blood, placenta, fetuses or uterine secretions or through consumption of contaminated raw animal products such as unpasteurized milk or cheese. Brucellosis is endemic in Iran. The aim of this study was to compare the molecular and serological tests for detection of brucellosis. In a cross-sectional study, the blood specimens were collected from 92 unvaccinated cattle during the time period September 2014 to July, 2015.. The serum samples were aliquoted and stored at −20°C until tested. The brucellosis diagnosis was established by indirect ELISA (iELISA), Rose Bengal, 2ME, Wright and polymerase chain reaction (PCR) method. In this investigation frequency of brucellosis by using Wright, 2ME, Rose Bengal, and iELISA methods were 42.3% (n= 39), 50% (n= 71), 78.2% (n=72), 75% (n=69), 86.2% (n=76), respectively. The specificity and sensitivity of iELISA was higher than the other tests. The 72 positive Rose Bengal samples in PCR were shown to be positive by both genes and 20 negative Rose Bengal samples were shown negative by both samples. Prevention of brucellosis mainly involves education, food quality and personal hygiene. Efforts should be made by responsible authorities. The results of this study showed specificity and sensitivity of indirect ELISA (iELISA) test is more than other methods. So, brucellosis diagnosis using of this test is recommended.
Keywords
Cattle; Brucellosis; PCR; serological tests
Download this article as:| Copy the following to cite this article: Saadat S, Mardaneh J, Ahouran M, Mohammadzadeh A, Ardebili A, Yousefi M. Mansouri M. Diagnosis of Cattle Brucellosis by PCR and Serological Methods: Comparison of Diagnostic Tests. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Saadat S, Mardaneh J, Ahouran M, Mohammadzadeh A, Ardebili A, Yousefi M. Mansouri M. Diagnosis of Cattle Brucellosis by PCR and Serological Methods: Comparison of Diagnostic Tests. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15204 |
Introduction
Brucellosis is a zoonotic disease, and an important public health problem in many parts of the world, especially in the Middle East region1. This bacterial disease is caused by various Brucella species, which mainly infect goats, sheep, cattle, swine, and dogs2. Brucellosis of cattle is a highly contagious disease caused by Brucella abortus and is characterized by abortion in late pregnancy and a high rate of infertility in herd1, 3. Humans generally acquire the disease through direct contact with infected livestock, consumption of contaminated dairy products, or by inhaling airborne agents in laboratories and slaughter houses1-3. The majority of human cases are caused by ingesting unpasteurized milk or milk products from infected goats, sheep or cattle3, 4.
More than 500,000 human brucellosis cases are reported from worldwide each year, but the number of undetected patients is believed to be considerably higher5. The traditional epidemiology of this zoonotic disease has changed dramatically over the last two decades, related to major political and socio-economic events. Thus while the incidence remains high in the Middle East and North African countries, it has been greatly reduced in Latin America and south European countries6.
The number of human cases is directly correlated with the number of infected animals within a defined region1, 7. Effective counteractions to reduce the incidence of human brucellosis are therefore based on continuous surveillance and control of livestock and pasteurization of animals products, and also proper cooking of all food origin and quality control of all such products5, 7. Once the brucellosis has been transmitted from its animal reservoir to humans, only early diagnosis and effective antibiotic therapy can prevent serious sequelae in patients8.
Successful eradication of brucellosis and control programs for domestic animals have been established in many countries around the world, but clinical presentation of the disease is nonspecific, and may be very atypical; therefore, laboratory confirmation by isolation or detection of specific anti-brucella antibodies is necessary for final confirmation of the disease. Because of the economic importance of cattle in developed and developing countries, means for B.abortus diagnosis and prophylaxis have been widely investigated, and several serological and non-serological tests developed for cattle brucellosis have been found useful for the diagnosis of brucella infection in animals9.
The aim of present study is to use serological (ELISA (iELISA), ELISA (ELISA), Rose Bengal, 2ME, wrigth) and molecular tests (PCR) for detection brucellosis in cattle and comparison results of this tests.
Materials and Methods
A total of 92 cattle were randomly selected in Shahryar region (Tehran, Iran). Herd size ranged from 2 to 10 animals and only animals greater than 6 months of age were selected. Both males and females were tested. None of the animals tested had received any vaccinations, nor was there any herd history of abortion. Blood samples of 10 ml were obtained using a sterile vacutainer tube from the jugular veins of the cattle and were divided into two tubes, the first containing the anticoagulant EDTA for PCR test, the other without anticoagulant for serum separation. The samples were transported on ice to the diagnostic laboratory of Tehran. Subsequently, Blood in plain tube was centrifuged at 6000 rpm for 5 min to obtained serum samples. The serum samples were aliquoted and stored at -20°C until tested.
Serological Techniques
The SERELISA®Brucella C-ELISA antibody test kit (Brucella OCB Ab mono indirect®SERELISA, France) was used to test the serum samples for antibodies to B. abortus according to the manufacturer’s instructions. The optical density (OD) values for each of the controls provided in the kit and serum samples in the wells were read at 450 nm using a microplate photometer (Universal Microplate Reader, Bio-Tek Instruments, Inc.). The percent inhibition (PI) values were calculated according to the manufacturer’s instructions.
PCR Method
DNA Extraction
DNA extraction from peripheral blood (PB) was performed using standard phenol-chloroform method. Briefly, five hundred microliters of PB was incubated for 24 hours at 56°C in 2 volumes of proteinase K lysis buffer (0.5% Tween 20, 0.5% Nonidet P-40, 10 mmol/L NaOH, 10 mmol/L Tris [pH 7.2], 320 g of proteinase K per milliliter) and boiled for 10 minutes. A simplified phenol-chloroform extraction was performed with 700 µL of this lysate, followed by ethanol precipitation and resuspension in 10 µL of sterile distilled water (DW).
PCR Assay
This consists of amplification of a 223-bp fragment from the gene coding for the synthesis of bacterial immunogenic protein (with a molecular mass of 31 kDa) on the external membrane of Brucella abortus (BCSP31). BCSP31 protein is specific to the genus Brucella and is present in all of its biovars. The amplification was performed with the primers B4 (59-TGG CTC GGT TGC CAA TAT CAA-39) and B5 (59-CGC GCT TGC CTT TCA GGT CTG-39) according to Kamal IH et al.10. All tests included positive controls of B. melitensis Rev-1 DNA and negative controls containing all of the reaction components except DNA. The gel agarose staining was performed with an ethidium bromide solution (0.5 mg/ml), and DNA bands were visualized under UV light using transilluminator system.
Statistical Analysis
Analysis of data was performed by using SPSS 16 software. Sensitivity, specificity, positive and negative predictive values, likelihood ratios, and 95% CIs were calculated using the Two-by-two 1.0 analyzer program (Robert M. Centor and Jerry Keightley).
Results
This study was carried out on 92 cattle suspected to be infected or had a history of brucellosis from Shahryar region (Tehran, Iran). Also these animals had no history of vaccination against brucellosis. Polymerase chain reaction and serological tests including Wright, Rose Bengal, iELISA, and 2ME were used in this study.
A total 92 specimens collected, Brucella spp. was detected in 76 (82.6%) of cases by polymerase chain reaction. Table 1 summarizes the results of Rose bengal, iELISA, 2ME, Wright and PCR tests. Analysis of the results revealed positive iELISA results in 76 cases (82.6%) and positive results for Rose bengal and 2-mercaptoethanol in 72 (78.2%) and 39 (42%) cases, respectively (table 1). A significant difference between the number of negative sera in 2ME and iELISA (53 to 16) and in 2ME and rose bengal (53 to 20) was found.
Table 1: Frequency of positive and negative cases of brucellosis by PCR and serological testes (wright, rose Bengal, 2ME and iELISA).
| Test | Positive (%) | Negative (%) | Total (%) |
| Wright | 46 (50) | 46 (50) | 92 (100) |
| Rose bengal | 72 (78.2) | 20 (21.8) | 92 (100) |
| 2-mercaptoethanol | 39 (42) | 53 (57.7) | 92 (100) |
| Indirect ELISA | 76 (82.6) | 16 (17.4) | 92 (100) |
| PCR | 76 (82.6) | 16 (17.4) | 92 (100) |
Tables 2 to 7 summarize the comparison of the results of rose bengal, iELISA and 2ME tests. Thirty nine (100%) iELISA-positive specimens displayed positive reaction via 2ME. Among the 76 (82.6%) iELISA-positive samples, 37 (69.8%) cases were negative by 2ME. iELISA results were negative in 39 (100%) 2ME positive cases (table 2). Among the 76 (82.6%) specimens that were positive by iELISA, 46 cases were positive by wright, among the 46 wright negative specimens in this group 16 cases (34.8%) were negative by iELISA. Out of the 46 wright-negative samples, 30 (65.2%) cases showed positive result with iELISA test (table 3).
Table 2: Comparison of 2ME and iELISA in testing of 92 cattle sera for brucellosis.
|
2ME |
iELISA | |||
| Positive (%) | Negative (%) | Total (%) | Correlation Coefficient | |
| Positive | 39 (100) | 0 (0) | 39 (42.4) |
0.268 |
| Negative | 37 (69.8) | 16 (30.2) | 53 (57.6) | |
| Total | 76 (82.6) | 16 (17.4) | 92 (100) | |
Table 3: Comparison of Wright and iELISA in testing of 92 cattle sera for brucellosis.
|
Wright |
iELISA | |||
| Positive (%) | Negative (%) | Total (%) | Correlation Coefficient | |
| Positive | 46 (100) | 0 (0) | 46 (50) |
0.348 |
| Negative | 30 (65.2) | 16 (34.8) | 46 (50) | |
| Total | 76 (82.6) | 16 (17.4) | 92 (100) | |
Among 72 rose bengal positive cases, 3 (4.2%) cases were negative by iELISA test, also from 20 rose bengal negative 7 cases were positive by iELISA (table 4).
Table 4: Comparison of Rose bengal and iELISA in testing of 92 cattle sera for brucellosis.
|
Rose Bengal |
iELISA | |||
| Positive | Negative | Total | Correlation Coefficient | |
| Positive | 69 (95.8) | 3 (4.2) | 72 (78.2) |
0.656 |
| Negative | 7 (35) | 13 (65) | 20 (21.8) | |
| Total | 76 (82.6) | 16 (17.4) | 92 (100) | |
Wright test failed to detect antibodies against Brucella in 26 cases from 46 cases which Brucella has been detected by rose Bengal test (table 5). Out of the 53 2ME-negative samples, 33 cases showed positive result with rose bengal test (table 6). Out of the 76 PCR positive samples, 7 cases showed negative result with rose bengal test (table 7). Polymerase chain reaction (PCR) method was used for detection of Brucella in samples. Brucella DNA was detected in 82.6% of specimens (Figure. 1).
Table 5. Comparison of Wright and Rose bengal in testing of 92 cattle sera for brucellosis.
| Rose Bengal | ||||
| Wright | Positive | Negative | Total | |
| Positive | 46 | 0 | 46 | |
| Negative | 26 | 20 | 46 | |
| Total | 72 | 20 | 92 | |
| Parameters | Estimate Percent | Lower – Upper 95% CIs | Method | |
| Sensitivity | 63.89% | (52.35, 74.02) | ||
| Specificity | 100% | (83.89, 100) | ||
| PPV | 100% | (92.29, 100) | Wilson Score | |
| PNV | 43.48% | (30.21, 57.75) | ||
| Accuracy | 71.74% | (61.81, 79.92) | ||
Table 6: Comparison of 2ME and Rose bengal in testing of 92 cattle sera for brucellosis.
| Rose bengal | ||||
| 2ME | Positive | Negative | Total | |
| Positive | 39 | 0 | 39 | |
| Negative | 33 | 20 | 53 | |
| Total | 72 | 20 | 92 | |
| Parameters | Estimate Percent | Lower – Upper 95% CIs | Method | |
| Sensitivity | 54.17% | (42.74, 65.17) | Wilson Score | |
| Specificity | 100% | (83.89, 100) | ||
| PPV | 100% | (91.03, 100) | ||
| PNV | 37.74% | (25.94, 51.19) | ||
| Accuracy | 64.13% | (53.95, 73.18) | ||
Table 7: Frequency of Brucella positive and negative cases diagnosed based on peripheral blood PCR and rose bengal test.
| PCR | ||||
| Rose Bengal | Positive | Negative | Total | |
| Positive | 69 | 3 | 72 | |
| Negative | 7 | 13 | 20 | |
| Total | 76 | 16 | 92 | |
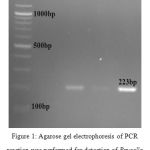 |
Figure 1: Agarose gel electrophoresis of PCR reaction was performed for detection of Brucella in animal samples. M= marker (100bp DNA ladder), 1= negative control; 2, 3,4, are positive specimens.
|
Discussion
Brucellosis is an important public health issue and as zoonotic disease in many developing and un-developed countries, including Mediterranean countries and countries on the Arabian Peninsula11. Brucellosis particularly caused by B. melitensis, is endemic in Iran, presumably affecting large numbers of animals as well as humans. It appears to be of particular risk in rural communities. Many improvements have been made for the diagnosis of brucellosis. However, problems exist with areas such as the diagnosis of latent infections.
Rapid and accurate diagnosis is fundamental for control and eradication of brucellosis12. Culture provides the definitive diagnosis of brucellosis and it is considered the gold standard method for it13. Because of difficulty of performing culture in the field, its consuming for the time, its health hazard and lack sensitivity of the most culture procedures, the serological tests are the main tools used for detection of Brucella infection in animals herds in diagnostic laboratories14.
In this study we evaluated the diagnosis of animal brucellosis by PCR compared with common serological tests. In our study, 82.6% of samples were positive based on the Indirect ELISA. In another study in the Saudia Arabia, the prevalence was reported 7.3% among 540 healthy subjects based on the IgG ELISA, thus, the authors might have underestimated the true prevalence of the disease15.
The sensitivity and specificity of ELISA in the present study (95.83% sensitivity and 65% specificity) are in agreement with those reported in other studies, for example, Mantur et al.16 reported an ELISA sensitivity of 71.3% and a specificity of 100%. PCR detects DNA which present in both living and dead Brucella organisms. In our study 82.6% of samples were positive by PCR. Our results are agreement with reports of Leal-Klevezas et al. that PCR has proved to be efficient in detecting the presence of Brucella spp. in blood17.
Since eradication of brucellosis from animals depends mainly on the rapid and accurate diagnosis of the infected animals, also eradication of brucellosis from human depends mainly on its eradication from the animals. Our data showed that seventy two samples are positive by rose bengal method, 39 samples by 2ME method and only 46 samples by Wright method. Although in this work the specificity of 2ME in comparison to Rose bengal was 100%, its sensitivity was 54.17%; in Erfanian and et al. report, the sensitivity of 2ME test was 93.7% (CI%95=91.1-95.6) 18.
A critical tool for the success of these measures is, without a doubt, an accurate and early diagnosis of the disease. The present research has compared the different diagnostic techniques (classical serological tests and PCR) and demonstrated the superiority of the latter technique for detecting small amounts of the pathogen in body fluids of infected animals.
In conclusion, PCR and indirect-ELISA offers a significant advantage over conventional serological methods in the diagnosis of brucellosis in endemic geographical region. The PCR test results can be particularly important in animals and human with clinical symptoms and signs, and negative serological tests, allowing the rapid confirmation of the brucellosis.
Control of brucellosis in livestock and humans depends on the reliability of the methods used for detection and identification of the causative agent. The disease can mimic many infectious and non infectious diseases. It is clearly important not only to detect but also to identify the species of Brucella implicated in natural infections. Since brucellosis is a zoonosis, the fight against this disease in humans and animals relies mainly on veterinary sanitation measures focused on the reduction or eradication of this disease in farm animals.
It should be also noted that in many cases, pathogenic organisms and antibiotic resistance bacteria are transmitted to humans from other sources including food animals, poultries, plants, fish, and other industries, in which antibiotics are used for different purposes and may lead to emerging resistant strains19-25. In developing countries, a combination of molecular methods (such as PCR) with one of the commonly used serological tests can be applied to detect brucellosis in cattle26. Animals herds and human food sources have to be included in national programs for control and eradication of Brucella and other food-borne bacteria (e. g., Salmonella spp., Enterobacter spp., E.coli, Staphylococcus aureus, Enterococcus spp.) in developing countries such as Iran19-25.
Acknowledgments
All co-workers listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. To the best of our knowledge, no conflict of interest, financial or other, exists.
Conflict of Interest
No conflict of interest exists.
References
- Gwida M, Al Dahouk S, Melzer F, Rösler U, Neubauer H, Tomaso H. Brucellosis- regionally emerging zoonotic disease? Croat Med J. 51(4):289-95 (2010).
CrossRef - Moges Woldemeskel. Zoonosis Due to Bruella suis with Special Reference to Infection in Dogs (Carnivores): A Brief Review. Open Journal of Veterinary Medicine. 3:213-21 (2013).
CrossRef - Parlak M, Güdücüoğlu H, Bayram Y, Çıkman A, Aypak C, Kılıç S, Berktaş M. Identification and determination of antibiotic susceptibilities of Brucella strains isolated from patients in van, Turkey by conventional and molecular methods. Int J Med Sci. 10(10):1406-11 (2013).
CrossRef - Salman MD, Meyer ME. Epidemiology of bovine brucellosis in the Mexicali Valley, Mexico: literature review of disease-associated factors. Am J Vet Res. 45(8):1557-60 (1984).
- Al Dahouk S, Sprague LD, Neubauer H. New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev Sci Tech. 32(1):177-88 (2013).
CrossRef - Boschiroli ML, Foulongne V, O’Callaghan D. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol. 4(1):58-64 (2001).
CrossRef - Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. 6(10):e1865 (2012).
CrossRef - Plumb GE, Olsen SC, Buttke D. Brucellosis: ‘One Health’ challenges and opportunities. Rev Sci Tech. 32(1):271-78 (2013).
CrossRef - Díaz-Aparicio E, Marín C, Alonso-Urmeneta B, Aragón V, Pérez-Ortiz S, Pardo M, Blasco JM, Díaz R, Moriyón I. Evaluation of serological tests for diagnosis of Brucella melitensis infection of goats. J Clin Microbiol. 32(5):1159-65 (1994).
- Kamal IH, Al Gashgari B, Moselhy SS, Kumosani TA, Abulnaja KO. Two-stage PCR assay for detection of human brucellosis in endemic areas. BMC Infect Dis.13:145 (2013).
CrossRef - Wernery U. Zoonoses in the Arabian Peninsula. Saudi Med J.2014; 35(12):1455-62.
- Mayada Gwida, Sascha Al Dahouk, Falk Melzer, Uwe Rösler, Heinrich Neubauer, and Herbert Tomaso. Brucellosis -Regionally Emerging Zoonotic Disease? Croat Med J. 51(4): 289-95 (2010).
CrossRef - Jacques Godfroid, Klaus Nielsen, and Claude Saegerman. Diagnosis of Brucellosis in Livestock and Wildlife. Croat Med J. 51(4): 296-305 (2010).
CrossRef - Tsegay A, Tuli G, Kassa T, and Kebede N. Seroprevalence and risk factors of brucellosis in abattoir workers at Debre Zeit and Modjo export abattoir, Central Ethiopia. BMC Infectious Diseases. 17:101 (2017).
CrossRef - Elsheikh AA, Masoud EE, Mostafa MF, Elkhawanky MM. Seroprevalence of 2 zoonotic diseases in Southwestern SaudiArabia: rift valley fever and Brucellosis. Saudi Med J. 32:740-41(2011).
- Mantur B, Parande A, Amarnath S, Patil G, Walvekar R, DesaiA, et al. ELISA versus conventional methods of diagnosingendemic brucellosis. Am J Trop Med Hyg. 83:314-18 (2010).
CrossRef - Leal-Klevezas DS, Martínez-Vázquez IO, López-Merino A, Martínez-Soriano JP. Single-step PCR for detection of Brucella spp. from blood and milk of infected animals. J Clin Microbiol. 33(12):3087-90 (1995)
- Erfanian M,Seyyed Nouzadi SM,Jarahi L. Evaluation of Diagnostic Sensitivity of Wright, Coombs Wright and 2-Mercapto Ethanol in Diagnosis of Brucellosis. Evidence-based Care Journal. 2(4): 69-74 (2013).
- Anvarinejad M, Pouladfar GR, Pourabbas B, Amin Shahidi M, Rafaatpour N, Dehyadegari MA, et al. Detection of Salmonella spp. with the BACTEC 9240 Automated Blood Culture System in 2008-2014 in Southern Iran (Shiraz): Biogrouping, MIC, and Antimicrobial Susceptibility Profiles of Isolates. Jundishapur J Microbiol. 9(4):e26505 (2016).
CrossRef - Mardaneh J, Soltan Dallal MM. Isolation and Identification Enterobacter asburiae from Consumed Powdered Infant Formula Milk (PIF) in the Neonatal Intensive Care Unit (NICU). Acta Med Iran. 54(1):39-43 (2016).
- Hassanzadeh P, Hassanzadeh Y, Mardaneh J, Rezai E, Motamedifar M. Isolation of Methicillin-Resistant Staphylococcus aureus (MRSA) from HIV Patients Referring to HIV Referral Center, Shiraz, Iran, 2011-2012. Iran J Med Sci. 40(6):526-30 (2015).
- Soltan Dallal MM, Mojarrad M, Baghbani F, Raoofian R, Mardaneh J, Salehipour Z. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Arch Iran Med. 18(3):167-72 (2015).
- Mardaneh J, Soltan-Dallal MM. Isolation and Identification of E. cowanii from Powdered Infant Formula in NICU and Determination of Antimicrobial Susceptibility of Isolates. Iran J Pediatr. 24(3):261-6 (2014).
- Shaghaghian S, Pourabbas B, Alborzi A, Askarian M, Mardaneh J. Vancomycin-Resistant Entrococci colonization in chronic hemodialysis patients and its risk factors in southern Iran (2005-2006). Iran Red Crescent Med J. 14(10):686-91 (2012).
- Mardaneh J, Soltan Dallal MM, Taheripoor M, and Rajabi Z. Isolation, Identification and Antimicrobial Susceptibility Pattern of Tatumella ptyseos Strains Isolated From Powdered Infant Formula Milk Consumed in Neonatal Intensive Care Unit: First Report From Iran. Jundishapur J Microbiol. 7(6):e10608 (2014).
CrossRef - Ducrotoy M, Bertu WJ, Matope G, Cadmus S, Conde-Álvarez R, Gusi AM, et al. Brucellosis in Sub-aharan Africa: Current challenges for management, diagnosis and control. Acta Trop. 165:179-193 (2017).
CrossRef







