Manuscript accepted on :April 04, 2017
Published online on: --
Plagiarism Check: Yes
Suha A. AL-Fakhar
Clinical Communicable and Infectious Diseases Research Unit /College of Medicine / Baghdad University, Baghdad – Iraq.
Corresponding Author E-mail: dr.alkarkhi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1184
Abstract
Circulating immune complexes(CICs) play an important role in many diseases in human. They can cause damage to kidney , joints, skin, and CNS through complement activation with or without local deposition.They can also modulate humoral and cellular immune response through binding to surface receptors of lymphocytes &phagocytes.CICs like activity has been reported in sera from patients with variety of infectious diseases caused by viral, bacterial, protozoal& helminthes. Total 25 patients with different problems of kidney and abortion history (14male&11female) and 25control groups(13 male&12 female).The CICs was detected by platelets aggregation test (Pl.A.test) and polymorphonuclus (PMN) were counted automatically. Polymorphonuclear cells depleted in number in patients with Toxoplasmosis 63.9 percentage and 77.4% percentage in control group ,there is no relationship between age and % of PMNs (P>0.001) in patients and control group ,13% of patients had CICs detected by PL.A. test and 4% of control group had CICs , There were no of patients had IgM of T.gondii and 8(32%)of them had IgG of T.gondii and 3(12%) of control group with IgM of T.gondii and 8% of them with IgG of T.gondii. PMN play an important role in the defense against toxoplasmosis and the clearance of CICs from circulation. Most of CICs formed in the circulation contained IgG of T.gondii, since most patients group of the present study did not have IgM of T.gondii but those with IgG of T.gondii containing IC and the number of PMNs was decreased in large number after T.gondii invasion.
Keywords
Immune Complexes; Leucocytes Polymorphonuclear;
Download this article as:| Copy the following to cite this article: AL-Fakhar S. A. Circulating Immune Complexes in Relation to Polymorphonuclear Leucocytes in Patients Infected With Toxoplasmosis. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: AL-Fakhar S. A. Circulating Immune Complexes in Relation to Polymorphonuclear Leucocytes in Patients Infected With Toxoplasmosis. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=14691 |
Introduction
Toxoplasma gondii, an intracellular coccidian, infects a wide range of eukaryotic cells and is an important opportunistic pathogen in humans and animals. The infection is frequently encountered without any symptom but there are two groups of high-risk individuals, the human fetuses and the immunosuppressed persons, particularly those with acquired immunodeficiency syndrome (AIDS), that frequently develop fatal toxoplasmic encephalitis (TE).1,2,3
Toxoplasmosis is a disease affecting 500 million people worldwide. The sero prevalence varies from 5% to 90% depending on geographical location, age, habit of eating raw meat or unwashed fruit and vegetables, and general level of hygiene. The incidence of infections is higher in warmer and humid cli- mate and increases with age. The disease can be congenital or acquired.4
Circulating immune complexes(CICs) play an important role in many diseases in human .They can cause damage to kidney, joints, skin, CNS through complement activation with or without local deposition.5
Circulating immune complexes(CICs) like activity has been reported in sera of patients with variety of infectious diseases caused by viral, bacterial, protozoal and helminthes agents.6 They can also modulate humoral &cellular immune responses through binding to surface receptors of lymphocytes.5
Antigen –antibody complexes can damage tissues by triggering inflammation. Recent studies have enabled the description of sequence of steps, which depend on intra- or perivascular location of complex formation. The lesions associated with perivascular complexes are characterized by plasma leakage and the recruitment of polymorphonuclear leukocytes.7
Presence of immune complex, associated with the absence of detectable levels of antibodies or free P30 can be an indicative of the stage of immune response to the infection. Predominance of immune complex in HIV positive samples may be associated with compromising of the mononuclear phagocytic system responsible for IC clearance from CSF. This could promote a longer persistence of the IC in this and other organic.8,9
A neutropenia is a risk factor associated with Aspergillus fumigatus, Candida albicans Mycobacterium tuberculosis and T.gondii infection.10,11,12,13,14,15,16,17,18 In some cases neutropenia has been correlated with impaired protective acquired immunity,suggesting that neutrophil function as immunomodulators of acquired immunity.16,17
Moreover, neutrophil–depeleted mice harbored an increased parasite burden. It was found that neutrophil depletion at the time of infection lead to development of lesions in multiple organs, including spleen ,lung,liver,and brain and was associated with an impaired ability to produce early gamma interferon (INF-Ɣ),tumor necrosis factor(TNF) andinterluki-12 . This is leading to fact that neutrophils are important immunomodulators early in the early course of T.gondii infection and play critical role in protecting the host from uncontrolled tachyzoite replication.19 In group of seropositive pregnant women with toxoplasmosis , CIC detection rates were noticeably higher in the samples showing both IgG and IgM antibodies.20
Both immunoglobulin G(IgG)and IgM were found in the CICs; however IgG was seen in the majority of sera were selected from patients with clinical symptoms generally associated with toxoplasmosis, more CICs were also again demonstrated.21
Circulating immune complexes (CICs) remained detectable for several weeks with non virulent strain of Toxoplasma, this period characterized by clinically healthy animals, indicating a subacute stage of the Toxoplasma infection. The positive CICs test requires great care but may provide useful information about the activity of a toxoplasma infection.22
The data suggest that phagocytosis of circulating immune complexes by neutrophils may interfere with the function of these cells in combating infection and also render them susceptible to removal from the circulation thus leading to the development of neutropenia.23
Materials and Methods
Total number of 50 Iraqi patients were included 25 patients with different problems of kidney and abortion history (14male&11female) and 25control groups(13 male&12 female). 5 ml of blood sample collected from each patients and control group under sterile .and the serum was divided into 2ml. for PMN counting and 3ml. of blood put in screw capped sterile plastic tube after centrifugation then the serum was divided in sterile appendrof tube each with 0.5ml. for platelet aggregations test(Pl.A.test)24)for detection CICs. The CICs was detected by platelets aggregation test (Pl.A.test) and polymorphnuclear (PMNs) were counted automatically. The study was carried out during the period from November / 2015 to February / 2016. All patients were obtained from those who had been admitted to / or attended the following health institution:
Renal Transplant Center / out patients clinic of Ghazi Al-Harriri hospital.
Baghdad Teaching Hospital/Gynecology and Delivery Department.
Laboratory of Blood Bank Center /Baghdad.
All laboratories tests were done in laboratories of Blood Bank Center.
ThePl.A.test test was done to supposed circulating immune complexes(CICs) in the sera of patients with toxoplasmosis. The platelets were used on the day of preparation , with made a pool of three lots of platelets .It was always recommended to decrease the effect of varying sensitivity of different lots of platelets from different donors.24,25 Platelets was counted using26 viable methods for platelets count are still used extensively which are valuable when the count is low. The diluents consist of 1% aqueous ammonium oxalate in which the red blood cells (RBCs) are lysed. There is a possibility that (RBCs) debris may be mistaken for platelets. The method is preferred to that using formal-citrate as diluents, which leaves the red cells intact and more likely to give incorrect results, when the platelets count is low. The method was done as following:
Two hundred (200µl) of platelets suspension was added to 0.38ml of diluting fluid (1%ammonium oxalate)in a small plastic tube.
The suspension was mixed with mechanical mixer or manually with Pasture pipette for 10-15 min .
The Neubauer counting chamber was filled with the suspension using a stout glass capillary or Pasteur pipette.
The counting chamber was placed in a moist Petridish and was left untouched for at least 20min .
The platelets when examined in the preparation under ordinary illumination appeared as a small highly refractile particles.
Calculation: Count of platelets/µl = N. × dilution ( diluent’s volume /volume counted µl) × 10(depth). N= number of platelets counted in an area of 1 mm².
If the number of platelets counted was high ,it is referable to use 2ml of 1% ammonium oxalate to 20µl of platelets suspension with consideration to dilution factor in the law above
The PL.A. test was performed using disposable microplates U shaped (cooke engineering Co. Alexandra, Virginia).
Two fold serial dilution of patients sera in PBS (pH =7.8)without glucose (1/2–1/4096) were made , using 25µl automatic micropipette. The sera were heated and inactivated at 56°C for 30 before use (24).
Then 25µl of platelets suspension (200,000 platelets/mm³ )was added to each well-containing diluted patients` serum or control sera.
The microtiter plate was agitated gently by side away movements for several minutes, then was covered with paraffin foil and incubated overnight at a temperature (5-8°C).
The sedimentation patterns were read in the following morning, using ordinary light microscope.
The Results Were Recorded as follows
Fully aggregated platelets were considered as positive result.
Not aggregated platelets were considered as negative result.
While not fully aggregate of platelets were considered as intermediate result.
The Results
The study included of 25 patients group (14male and 11 female) infected with toxoplasmosis and with different diseases(e.g. R.T.,N.S.and SLE) and 25 control healthy group(13, the mean of ages of patients group was (35.4±13.72) and (34.6±4.34)of control group (Table-1-).The mean of percentage of PMN was(63.91±11.39)of patients group and (77.48±8.89)of control group. There was no significant differences between the means of ages of patients and control groups(P>0.001).
Table 1: Age of patients with toxoplasmosis and control group in relation with PMNs percentages.
| Groups | N | Mean | Std. Deviation | Std. Error Mean | |
| Age | Patient | 25 | 35.4000 | 13.72346 | 2.74469 |
| Control | 25 | 34.6000 | 4.34933 | .86987 | |
| PMN% | Patient | 25 | 63.9120 | 11.39694 | 2.27939 |
| Control | 25 | 77.4840 | 8.89933 | 1.77987 |
Table-2-showed the prevalence of CICs in patients and control groups ,there was 13(68.4%)patients with CICs in their sera and 6(31.6%)patients without CICs in their sera, while there was 4(16%) of control group with CICs in their sera and 21(84%)without CICs in their sera. There was a significant differences between their prevalence of CICs in the sera of the groups (P<0.0001).
Table 2: Prevalence of Circulating Immune Complexes in Patients with Toxoplasmosis and control groups
|
Groups |
CiCs | Total | ||
| +ve | -ve | |||
| Patient | Count | 13 | 6 | 19 |
| % within Group | 68.4% | 31.6% | 100.0% | |
| Control | Count | 4 | 21 | 25 |
| % within Group | 16.0% | 84.0% | 100.0% | |
| Total | Count | 17 | 27 | 44 |
| % within Group | 38.6% | 61.4% | 100.0% | |
Table -3-Showed the there was no patient with IgM of T.gondii ,while there was 25(100%) of patients with IgM of T.gondi. comparison control group there was 3(12%) with IgM of T.gondi ,while there was 22(88%) control group without IgM of T.gondi, there was no significant differences between patients and control group P>0.01.
Table 3: Prevalence of IgM in patients with Toxoplasmosis in comparison to healthy control
| Groups | T.gondii IgM | Total | ||
| +ve | -ve | |||
| Patient | Count | 0 | 25 | 25 |
| % within Group | .0% | 100.0% | 100.0% | |
| Control | Count | 3 | 22 | 25 |
| % within Group | 12.0% | 88.0% | 100.0% | |
| Total | Count | 3 | 47 | 50 |
Table-4-There was 8(32%) of patients with IgG of T.gondii ,while there was 17(68%) of patients without IgG of T.gondii. In control group there was 12(48%) healthy persons had IgG-Abs. of T.gondii ,while there was 13(52%) healthy persons without IgG of T.gondii. There was no significant differences(P<0.05) between patients and control groups P>0.248.
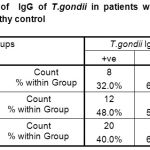 |
Table 4: Prevalence of IgG of T.gondii in patients with Toxoplasmosis in comparison with healthy control
|
Table (5) showed the prevalence of circulating immune complexes (CICs)detected by PL.A. test and polymorphonuclear cells(PMNs) in patients group. There was 16 patients with CICs in their sera the mean of their ages was (35.1±16.402), and there was 9 patients without CICs in their sera the mean of their ages was(35.55±7.77).There was 16patients with PMNs% (64.268±10.39),while there was 9 patients without CICs their sera and PMN% was (63.27±13.65).There was no significant P>0.840. differences between the two groups
Table 5: The prevalence of circulating immune complexes detected by Platelets Aggregation Test and polymorphonuclus cells(PMNs) in patients with toxoplasmosis and control.
| Circulating immune complexes detected by Platelet aggregation test | N | Mean | Std. Deviation | Std. Error Mean | |
| Age | +ve | 16 | 35.3125 | 16.40211 | 4.10053 |
| -ve | 9 | 35.5556 | 7.77996 | 2.59332 | |
| PMN% | +ve | 16 | 64.2688 | 10.39447 | 2.59862 |
| -ve | 9 | 63.2778 | 13.65209 | 4.55070 | |
There was 3(18.8%) patients with T.gondii IgG Abs (Table-6) and CICs detected PL.A. test in their sera,while there was 5(55.6%)patients without T.gondii IgG and CICs detected PL.A. test in their sera. There was no significant differences P>0.058 between the two group of patients and control.
Table 6: Correlation between the presence of T.gondii IgG Abs. and CICs detected by PL.A.test.
|
CICs detected by Pl.A.test |
T.gondii IgG | Total | ||
| +ve | -ve | |||
| +ve | Count | 3 | 13 | 16 |
| % within CiCs PL.A. | 18.8% | 81.3% | 100.0% | |
| -ve | Count | 5 | 4 | 9 |
| % within CiCs PL.A. | 55.6% | 44.4% | 100.0% | |
| Total | Count | 8 | 17 | 25 |
| % within CiCs PL.A. | 32.0% | 68.0% | 100.0% | |
Discussion
Toxoplasmosis is caused by a coccidian parasite Toxoplasma gondii. It is world-wide in distribution and infects most of the vertebrates. The Felides are its definitive host. The humans are infected either through contaminated food, water, transfusion of infected blood, organ transplantation or from mother-to-foetus through placenta.27 Toxoplasma gondii is an obligate intracellular protozoan parasite that infects at least a third of the world’s population. Infection with the parasite is divided into a limited acute stage. Followed by a persistent chronic stage. In the chronic stage of T. gondii forms cyst, found mainly in brain and muscle tissues, which can persist for the lifetime of the host.28
Table(1)showed that patients with low percentage of PMNs 63.9% ,in comparison with control group 77.4 % of PMNs .The results agreed with that reported by Salyes,199929 that the previous studies showed neutrophil was play an important role in resistance to acute primary T.gondii infection and that depletion of neutrophils reduces the numbers of CD4+ and CD8+ lymphocytes recoverable from peripheral blood of infected but not uninfected mice. Also, it was found that examined the development of immunity during T. gondii infection in mice depleted of neutrophils by monoclonal antibody (MAb) administration. T. gondii.19
Table- 2- showed that 13% of patients with CICs in their sera in comparison with 4% of healthy group had CICs in their sera ,the results compatible with reported by Sonia,20057 management the deposition of immune complexes in tissues consider the pathogenic mechanism underlying a variety of human diseases. Also, Gladkova et.al.,200020 described that circulating immune complexes(CICs) in the host just in early T.gondii invasion can be present in the blood. In addition, there has been no report of CIC in patients with toxoplasmosis. Some aspects of toxoplasmosis, notably the occasional occurrence of glomerulonephritis or congenital nephrotic syndrome30 suggest a pathogenic role for CIC.5
The results included ( Table-3- and Table-4-)no of patients with IgM of T.gondii but 8(32%)of them with IgG of T.gondii, while 3( 12%) of control group with IgM of T.gondii and 12(48%)of them with IgG of T.gondii and the results suggested that patients might be chronic phase of toxoplasmosis and might IgG Abs of T.gondii form CICs in there circulation ,while control group might had active phase of toxoplasmosis since they with both IgM and IgG of T.gondii in there sera and there antibodies did not form immune complexes in their circulation. These results in line with Ghasemian et al.,200731 who reported that the disease in immunocompromised individuals such AIDS, transplant recipients,persons receiving immunosuppressive drugs usually is due to reactivation of latent infection but can result from acute infection. Toxoplasmosis in these persons leads to lethal meningoencephalitis,focal lesions of the CNS, and less commonly,myocarditis or pneumonitis.
Also, IgG antibodies to Toxoplasma are usually present 1-2 weeks after acquisition of the infection and usually persist for life. For immunocompetent persons, seroconversion with high concentrations of Toxoplasma specific IgM and a 4-fold increase in specific IgG titer is indicative of recent infection. It has been known that 15-58% of humans are infected with T.gondii, but the rate of infection varies widely by location, age and other factors31,While, Wongkamchai et al.,199532 mentioned that Toxoplasma-specific IgG and IgM antibodies were determined in healthy persons and patients with different symptoms who were suspected of toxoplasmosis. Specific IgG were detected in 3.2% of healthy persons, 12.5% of patients with ocular disease and 42.5% in HIV positive patients. Only 3.1% of patients with ocular disease were positive for specific IgM Ab. No specific IgM were found in the other samples studied .
Table-5- showed that there were 16 patients with circulating immune complexes and with the percentage of PMNs was (64±10.26),while there were only 9 of patients without CICs in their circulation.
Also, these results compatible with that reported by Steffelaar et al.,197633 who reported that antigen-antibody complexes are eliminated from the circulation by phagocytosis effected in the reticuloendothelial system (RES) and by deposition in renal glomeruli and other tissues.34 In addition, neutrophils have long been regarded as one of the most important of the induced host innate defenders primarily because they are the earliest cells to arrive at sites of infection or inflammation in response to chemotactic signals to eliminating invading pathogens.35
Table (6)showed that there 3(18.8%) patients with immune complexes(CICs) and with IgG of T. gondii and the result agree with Gladkova et al.,200020 who reported that CICs in serum of pregnant women exhibit only IgG contained mainly T.gondii Ag having MW of 67and 30 KD.
Finally deposited IC s cannot be detected by PL.A. test, if only when they are found in soluble form in cases of antigen excess, since PL.A. test detect IgG containing ICs , whether or not they fix complement but does not detect complexes formed with IgM .Also PL.A. test reaction caused by ICs are competitively inhibited by some rheumatoid factor,C1q and to lesser degree by monomeric IgG36 The final diagnosis of toxoplasmosis depends on the results of ELISA method that was used for the detection of IgM Toxoplasma antibodies chosen in the decision of the final diagnosis of toxoplasmosis and not IgG , because acute toxoplasmosis is usually diagnosed on the basis of IgM antibody detection.37
References
- Flávia A. C.,Maria A. S., Deise A. O. S., Lioyd H. K and José R. M. Detection of Toxoplasma gondii soluble antigen, SAG-1(p30), antibody and immune complex in the cerebrospinal fluid of HIV positive or negative individuals. Inst. Med. trop. S. Paulo. 1999;41:6 .
- Navia B. A.,Petito C. K.,Gold J. W. M., Cho E. S., Jordon B. D.,Price J. W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19:224–238.
CrossRef - Remington J. S.,Toxoplasmosis G. D., In: Remington J. S., Klein J. O., editors. Infectious diseases of the fetus and newborn infant. W. B. Philadelphia, Pa: Saunders Co. 1990;89–195.
- Al-Jebouri M.,Al-Janabi M.,Ismail H. The prevalence of toxoplasmosis among female patients in Al-Hawija and Al-Baiji Districts in Iraq. Open Journal of Epidemiology. 2013;3:85-88.
CrossRef - Siegel J. P and Remington J. S. Circulating immune complexes in toxoplasmosis detection and clinical correlates. exp.Immunol. 1984;52:157-163.
- Theofilopulos A .N.,Rubin R., Balderas R.,Tsokos G.,Tan E and Dixon F. J. Monoclonal mouse and human rheumatoid factors with multiple autoantigen specificities.Proc.,FedAm.Soc.Exp.Biol. 1984;43:1735(Abstract).
- Sonia J., Mariano S. C. Immune complexes –mediated tissue injury a multistep paradiagm, Trends in Immunology. 2005;26:48-55.
CrossRef - HELLERBRAND C., GOEBEL E. D and DISKO R. High predictive value of Toxoplasma gondii IgG antibody levels in HIV-infected patients for diagnosis of cerebral toxoplasmosis. J. clin. Microbiol. infect. Dis. 1996;15:869-872.
- HOLLIMAN R. E. Toxoplasmosis and the acquired immune deficiency syndrome. Infect. 1988;16:121-128 .
- Barteneva N., Theodor I., Peterson E. M.,de la Maza L. M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833.
- Bliss S. K., Butcher B. A.,Denkers E. Y. Rapid recruitment of neutrophils with prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521.
CrossRef - Bliss S. K., Zhang Y., Denkers E. Y. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J Immunol. 1999;163:2081–2088.
- Conlan J. W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635.
- Conlan J. W.,North R. J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. Exp. Med. 1994;179:259–268.
CrossRef - Mehrad B., Moore T. A.,Standiford T. J. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. Immunol. 2000;165:962–968.
CrossRef - Pedrosa J.,Saunders B. M.,Appelberg R.,Orme I. M., Silva M. T.,Cooper A. M. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583.
CrossRef - Romani L., Mencacci A.,Cenci E., Del S. G., Bistoni F.,Pff P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362.
- Watanabe K., Noda K.,Hamano S.,Koga M.,Kishihara K., Nomoto K., Tada I. The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol. Res. 2000;86:188–193.
CrossRef - Susan K.,Bliss L.,Cristina G., Ana A and Eric Y. D. Neutrophil Depletion during Toxoplasma gondii Infection Leads to Impaired Immunity and Lethal Systemic Pathology. Infect Immun. 2001;69:4898–4905.
CrossRef - Gladkova S. V.,Bormotov N. I.,Dedkova L. M. Composition of circulating immune complexes in acute toxoplasmosis. Med-parazitol-(Mosk) . 2000;4:15-8.
- Rotha A.,Van-Knapen F.,Baarsma G. S.,Kruit P. J.,Loewer-Sieger D. H.,Kijlstra A. Serology in ocular toxoplasmosis. J. Ophthamol. 1986;70:615-22.
- Van K.,Panggabean S. O., Van-Leusden J. Demonstration of Toxoplasma antigen containing complexes in active toxoplasmosis.Clin-Microbiol. 1985;22(4):645-50.
- Hurd E. R., Andreis M., Ziff M. Phagocytosis of immune complexes by polymorphonuclear leucocytes in patients with Felty’s syndrome.). Clin Exp Immunol. 1977;28:413-25.
- Penttinen K., Vaheri A and Myllyllä G. Detection and characterization of immune complexes by platelets aggregation test /immune complexes formed in vitro.Exp.Immunol. 1971;8:389-397.
- Melartin L.,Myllylä G and Penttinen K. Detection of Au (1) Antigen by immunodiffusion and platelets aggregation tests. Vox San. 1970;19:239-245.24.
- Dacie S.V and Lewis S. M. (eds.) Practical Haematology,17th ed. Churchill Livingstone, Edinburgh. 1991.
- Singh S. Mother-to-child transmission and diagnosis of Toxoplasma gondii infection during pregnancy . J. Medical Microbio. 2003;21:69-76 .
- Carolyn R. S and Felix Y. Complex Immune Cell Interplay in the Gamma Interferon Response during Toxoplasma gondii Infect Immun. 2014;82:3090–3097.
CrossRef - Salyes P. C.,Johnson L. L. Exacerbation of toxoplasmosis in neutrophil-depleted mice.Immu. 1999;15:249-58.
- Shahin P. J. Congenital nephrotic syndrome associated with congenital toxoplasmosis. Pediatr. 1974;85:366-70.
CrossRef - Ghasemian M., Sh M.,Saki J.,Pedram M. Determination of Antibodies (IgG, IgM) against Toxoplasma gondii in Patients with Cancer.Iranian J. Parasitol. 2007;2:1-6.
- Wongkamchai S.,Rungpitaransi B.,Wongbunnate S., Sittapairochana C. Toxoplasma infection in healthy persons and in patients with HIV or ocular disease. Southeast Asian J. Trop. Med. Public Health. 1995;26:655-8.
- Steffelaar J. W.,Claire B., Graff-Reitsma D. E and FeltkampvroomM. T. Immune complex detection by immunofluorescence on peripheral blood polymorphonuclear leucocytes . exp. Immunol. 23:272-278.
- Tanya N.,Tsokos G. M.,Tsokos C and Naotake T. Mechanism of IC mediated neutrophil recruitment and tissue injury.Circulation. 2010;120:202-2024.
- Delbert S.,Abi A., Changyou L.,Carissa J.,Ball C.,Michael R., King C.,Gerald E.,Duhamel B and Denkersa Y. E. Toxoplasma gondii Triggers Release of Human and Mouse Neutrophil Extracellular. Traps, Infect Immun. 2012;80:768–777
CrossRef - Cochrance C. G. Detection of immune complexes in the circulation. In: Mathov E., Sndo T., Narajo P.(eds.).Mechanisms of immunopathology. Excerpta Medica, Amesterdam, Oxford. 1979;212.
- Salman S. L and Juma A. S. M. Correlation between apoptosis and Toxoplasma in abortion induction :Relevance of TUNEL assay. European J. Scin. Res.2009;37:406-425.







