Sukohar Asep1, Herawati Hening2, Sari Gema P3, Setiawan Gigih1, Morfi Chicy Widya1 and Sahidin4
1Faculty of Medicine, Lampung University, Lampung 35145, Indonesia.
2Department of Research and Development, Dharmais Cancer Hospital, Jakarta 11420, Indonesia.
3Departemen of Immunology Faculty Of Medicine, Indonesia University, Jakarta 10430, Indonesia.
4Faculty of Pharmacy, Haluoleo University, Kendari 93232, Indonesia.
Corresponding Author E-mail: drgigih88@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1154
Abstract
Jatrophone is an isolated compound from Jatropha gossypifolia’s sterm bark plant which have cytotoxic activities against human cell lines cancer. This project studied about cytotoxic evaluation of jatrophone against human cell line liver cancer Hep G2 1886. We also aim to discover jatrophone as new anticancer agent against hepatocellular carcinoma. The potential anticancer activity was measured using cultured cancer human cell line Hep G2 from Tohuku Uiversity of Japan. And the cytotoxic properties of jatrophone toward human cell line liver cancer Hep G2 1886 was evaluated using MTT assays. The results of the study are cytotoxic potency of jatrophone were indicated by IC50 value against cell line Liver Cancer Hep G2 1886 is 3.2 µM, meanwhile IC50 value of jatrophone against another cell lines cancer such as human colon cancer line WiDr, cervix cancer cell line HeLa and stomach cancer AGS are 8.97, 5.13 and 2,5 µM respectively. Jatrophone had better anticancer potency activites against cell line liver cancer Hep G2 than WiDr and HeLa cell lines cancer. In addition jatrophone also had better anticancer activity against cell line cancer liver Hep G2 than standard anticancer such as sorafenib and ATO (Arsenic Trioxyde).
Keywords
Hep G2 1886; Jatrophone; MTT{3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide} assay
Download this article as:| Copy the following to cite this article: Asep S, Hening H, Gema S. P, Gigih S, Widya M. C, Sahidin S. Anticancer Activity of Jatrophone an Isolated Compound from Jatropha Gossypifolia Plant Against Hepatocellular Cancer Cell Hep G2 1886. Biomed Pharmacol J 2017;10(2). |
| Copy the following to cite this URL: Asep S, Hening H, Gema S. P, Gigih S, Widya M. C, Sahidin S. Anticancer Activity of Jatrophone an Isolated Compound from Jatropha Gossypifolia Plant Against Hepatocellular Cancer Cell Hep G2 1886. Biomed Pharmacol J 2017;10(2). Available from: http://biomedpharmajournal.org/?p=15434 |
Introduction
Almost any kind of vegetation can grow in Asia, and most of it has been used by Asian ancestors as a traditional medicine to treat many different type of disease (1). In Indonesia there are up to 90.000 kind of medicinal plants which 9.600 of it already used as herbal medicine with different formulas and indications (2). Especially in Lampung Province which have a lot of agrarian resources , there is a wide variety of plants that have been studied as traditional medicinal plants such as coffee robusta (3). Other than robusta coffee, there are other plants that are known and used as traditional medicine i.e Jatropha curcas plants (barbados nut) and Jatropha gossypifolia (bellyache bush). research shown that both have potential effects as a traditional medicine on the stem, seeds and leaves (4).
The name of genus Jatropha, which is a family of Euphorbiaceae, is derived from Greek word iatros meaning physician and trophe which mean food and closely connected as its/ function as a medicinal plant (5). In India, some parts of the bellyache bush that can be used as medicinal plant were the resin that has effect as a potential anticancer and its roots that can be used as antidote to snake bite venom. In addition the leaves, seeds, stem and twig is also believed to have analgetic effects, antimicrobial, antiinsect, antifungal, anticancer and anti inflammation agent (6). In Indonesia, the part of bellyache bush commonly used as traditional medicine were resin, leaves, fruit and seeds. Indonesian citizens using those parts to treat variety of complaints such as vaginal discharge, ear inflammation, toothache, mouth ulcer, flatulence-cold, constipation, fungi, swelling, sores, bleeding, rheumatism, cough and deciduous phlegm (7).
Main metabolite found from bellyache bush was terpenoids compounds. One of the derivate of these terpenoids compounds, namely deterpenoid have a chemical structure in the form of phenolics, flavonoids and saponins that functions as an antioxidant, anticancer and antiinflamation. This compound was found in root, stem and resin plants (8).
Jatrophone, curcusone B and jatropholone are three diterpenoid compounds which have been successfully isolated from both Barbados nut and bellyache bush. Jatrophone isolated from stem of bellyache bush while curcusone B and jatrophalone A were from the stem of Barbados nut (9). Those compound has activity as antibacterial, antifungal and anticancer (10). Jatrophone is diterpenoid compound with chemical bond cluster C20H24O3 (11). Jatrophone, a diterpenoid isolated from the stem of bellyache bush, was believed to have significant cancer growth inhibition activity(9).
Cancer is currently the main cause of death in the world, both of in developed and developing country (12). In the world as much as 14.1 million new patients suffering from cancers found annually and 8.2 million people die annually caused by cancer (12). The top five of cancer which causes death was lung cancer, breast cancer, colon cancer, liver cancer and gastric cancer (13). The risk of cancer have been increased, caused by the lifestyle and behavior in the form of smoking, lack of fruit and vegetable consumption, less physical activity and reproductive changes in the form of a low birth rate and old age pregnancy (14). Based on the number of risk factors can be estimated an annual cancer cases will rise from 14.1 million from 2012 to 22 million in 2032 (14).
The number of cancer incidence in Indonesia by year 2013 amounting to 1.4% of the whole population or as many as 347,792 people with the largest population in Yogyakarta, Central Java and East Java province. Risk factors that cause the high numbers of cancer incidence in Indonesia are poor eating style and behaviour in the form of lack of vegetable and fruit consumption, smoking, obesity and the consumption of foods contain high fat (14).
Hepatocellular cancer in top 5 large number of whole cancer cases and increase amount 500.000 new cases per year (15). While in Indonesia, according to the data from installation of early detection and health promotion Dharmais Cancer Hospital 2010-2013 hepatocellular cancer is in the top 10 cancer incidence in Indonesia (14). Hepatocelluler cancer in Indonesia tends to increase every year. It is associated with endemic hepatitis B and hepatitis C disease in Indonesia. Another risk factor is the habit of drinking alcoholic beverages that can cause the onset of liver cancer at a young age (16).
The treatment for liver cancer is a high cost expenditure because chemotherapy drugs for liver cancer is very expensive. In Taiwan, cost needed for one package of chemotherapy drug sorafenib is amount to USD 16,280 for each patient (17). In China, one patient must pay USD 1.331,65 up to USD 4.524,33 to purchase the drug sorafenib (18). In Indonesia for the treatment of hepatocellular carcinoma in type A hospital according to the INACBGS (Indonesia Case Base Groups) is provided funding in the amount of IDR 15 million for all treatments (19).
Rapid development of science and technology, as well as the high cost for current treatment of hepatocellular cancer and growing interest of researchers towards the discovery of new active compounds for healing hepatocellular cancer, we conduct research to discover the new anticancer compounds from plants and herbs that can be effective as anticancer drugs, inexpensive and have a good anti-cancer effectivity. This is an invitro anticancer activity study to measure active compounds of jatrophone, curcusone B and the jatropholone A against cancer cell Hep-G2 1886.
This research using hepatocelluler cancer line Hep G2 1886 because it has octreotide antiproliferation effect. Octreotide in medicine has been used as a therapy for neuroendocrine tumors and pituitary tumor. In a recent study with humans, it has been proven that giving octreotide as hepatocellular carcinoma therapy lowers death rate due to cancer. From that moment, variety of research begin using human cell line hepatocellular carcinoma Hep G2, although some of the research data is still controversial. Hep G2 used in this study isolated from hepatocarcinoma cell because this cancer represent 5% of all cancer incindence and estimated more than 500.000 new case of hepatocelluler cancer occur each year (20).
Jatrophone has been widely studied invitro to asses its cytotoxic effect rate against human cancer cell line in vitro i.e. assessment of jatrophone effect against P 388 lymphocytic leukemia-, KB cell lines, Eagle’s nasopharynx carcinoma, Lung fibroblasts. it also has the effect of antiproliferation against fibroblasts cells CCL-171, AGS CRL-1739, HTB-58, HTB-1 bladder, and CCL-240 leukemia cells (21). Sahidin et al has reported that jatrophone have better cytotoxic potential than other compounds like curcusone B and the jatropholone A against HeLa cell lines WiDr and even better against standard anticancer drugs such as tamoxifen and doxorubicin (9). Cell culture technique of hepatocellular carcinoma cell line Hep G2 1886 was used in this study and to assess the cytotoxic activity of jatrophone, we use the default value of the inhibitor concentration 50 (IC50) for these compounds added invitro in varying concentrations, so that we can assess the anticancer effect of jatrophone against cell line Hep G2 1886.
Materials and Methods
Preparation and culture Cell Line Hep G2 1886
This study done in the laboratory of research and development of Dharmais Cancer Center Hospital Jakarta in March 2016 until January 2017. Research using cell culture techniques of cell line hepatocellular carcinoma Hep G2 1886 from Tohuku University of Japan. Cell Line Hep G2 1886 cultured in a Flask T75 using DMEM (Dulbecco’s Modified Eagle Medium) (Sigma D5671) complete medium contains 10% FBS (Fetal Bovine Serum) (Himedia RM9955). Cells harvested after 80% confluent using trypsinization method and seeded in 96-well plate with the density 2 x 105 cells/well. Cells cultured in 100 µl DMEM complete medium contains 10% FBS for 24 hours in an incubator at 37°C with 5% CO2
Next, the medium was replaced with DMEM medium without FBS, cells incubated again for 6 hours in an incubator at 37 ° C with 5% CO2 to synchronize the cell cycle. After the medium without FBS DMEM has been disposed, the cell ready to be given preferential treatment.
Jatrophone Active Compounds
 |
Figure 1: Chemical structure JatrophoneC20H24O3(11) Click here to View figure |
Jatrophone active compounds that has been purified dissolved in DMSO (Sigma Aldrich M81802) as stock solution at 80 mg/ml.
Next, the medium was replaced with DMEM without FBS, cells incubated again for 6 hours in an incubator at 37 ° C with 5% CO2 to synchronize the cell cycle. After the medium without FBS DMEM has been disposed, the cell ready to be treat by serial dilution of jatrophone from various concentration from 5-0,156 ug/ml. For the treatment, jatrophone diluted by serial dilution method (diluted in DMEM medium containing 1% FBS) to be treat to Hep G2 cell line 24 hours. 1% FBS used on the medium to minimised the binding between the drugs and the protein on the FBS. The final concentration of DMSO (Dimethyl Sulfoxide) in each sample did not exceed 1% v/v, to keep the cytotoxicity of DMSO at less than 10%. Each well washed with PBS (Phosphate Buffered Saline) twice before the medium change with DMEM containing MTT (Sigma Aldrich M2128).
MTT Assay
Method of MTT assay was performed to find out the percentage of cells that are still alive after being given the treatment with active compounds. After that MTT were dissolved with PBS until its concentration reaches a concentration of 5 mg/ml. MTT concentrates then dissolved in the medium of complete DMEM containing 10% FBS so that the final concentration of MTT is 0.5 mg/ml. 100 µl MTT reagent are added into each of the wells. the cells are incubated for 4 hours in the CO2 incubator. After purple crystals of formazan formed, added 100 µ l of DMSO to each wells. 96-well plate wrapped with aluminum foil and incubated for 4 hours at room temperature.
Next 96-well plate read at 595 nm wave length (550-600 nm) with Biorad Microplate reader Model 680. Formula to count % viable cells : (treatment OD – blank OD) / (control OD – blank OD) x 100.
Calculation of IC50
IC50 calculate by method that developed by Miller and Tainter (22), Litchfield and Wilcoxon (23), Weil (24), and Finney (25). Percentage of the death cell which calculate fro MTT Assay result then transformed into probit, or probability versus log concentration. % death cell count by formula : 100 – % viable cells.
Table 1: Transformation of percentage mortalities to probits
| % | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|
0 |
– |
2,67 |
2.95 |
3.12 |
3.25 |
3.36 |
3.45 |
3.52 |
3.59 |
3.66 |
| 10 | 3.72 | 3.77 | 3.82 | 3.87 | 3.92 | 3.96 | 4.01 | 4.05 | 4.08 | 4.12 |
| 20 | 4.16 | 4.19 | 4.23 | 4.26 | 4.29 | 4.33 | 4.36 | 4.39 | 4.42 | 4.45 |
| 30 | 4.48 | 4.50 | 4.53 | 4.56 | 4.59 | 4.61 | 4.64 | 4.67 | 4.69 | 4.72 |
| 40 | 4.75 | 4.77 | 4.80 | 4.82 | 4.85 | 4.87 | 4.90 | 4.92 | 4.95 | 4.97 |
| 50 | 5.00 | 5.03 | 5.05 | 5.08 | 5.10 | 5.13 | 5.15 | 5.18 | 5.20 | 5.23 |
| 60 | 5.25 | 5.28 | 5.31 | 5.33 | 5.36 | 5.39 | 5.41 | 5.44 | 5.47 | 5.50 |
| 70 | 5.52 | 5.55 | 5.58 | 5.61 | 5.64 | 5.67 | 5.71 | 5.74 | 5.77 | 5.81 |
| 80 | 5.84 | 5.88 | 5.92 | 5.95 | 5.99 | 6.04 | 6.08 | 6.13 | 6.18 | 6.23 |
| 90 | 6.28 | 6.34 | 6.41 | 6.48 | 6.55 | 6.64 | 6.75 | 6.88 | 7.05 | 7.33 |
Table 2 : The percentage of the number of viable cells
| Dose (µg/ml) | Log Dose | % Viable Cell | % Death Cell | Probits |
| 5 | 0,699 | 47,525 | 52,475 | 5,05 |
| 2,5 | 0,398 | 48,535 | 51,465 | 5,03 |
| 1,25 | 0,097 | 53,586 | 46,414 | 4,9 |
| 0,625 | -0,204 | 53,788 | 46,212 | 4,9 |
| 0,313 | -0,505 | 54,192 | 45,808 | 4,87 |
| 0,156 | -0,806 | 59,444 | 40,556 | 4,75 |
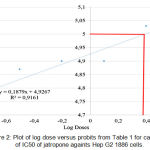 |
Figure 2: Plot of log dose versus probits from Table 1 for calculation of IC50 of jatropone againts Hep G2 1886 cells. |
Result
IC50 Jatrophone Againts Cell LineHep G2 1886
Table 2 shows that on dose range between 50 – 0,156 ug/ml, Jatrophone inhibited the diferentiation of Hep G2 1886 cells. It also shows that the IC50 was on range between 1,25 – 2,5 ug/ml. The percentage of death cells transformed into the probits score as shown on Table 1. From the log dose versus probits on Fig. 2, it shows that as an anticancer candidate, jatrophone has IC50 1,31 ug/ml (3,2 uM). From NCI USA standardisation, compound that has IC50 0,0-2,0 ug/ml is categorised as a highly active compound for cancer.
Discussion
Anticancer drugs generally have a narrow therapeutic window (26). Therefore on the research of the development of new anti-cancer drugs needed research cell present so that we can assess the lowest drug concentration had an effect of therapy (27). The lower the concentration of an active compound of anticancer drugs therapeutic effect conferring signifies a high selectivity in such compounds against targets cancer cells(28). Therefore current research many active compounds invitro done to human cell line so that we will get the concentration of the active compound of anticancer drugs lowest effect therapy. An in vitro IC50 calculation is a very basic starting point in determining the potential efficacy of a developmental drug and can be used as the most simple method to measure the pharmacokinetic because it is focusing on the drug and the target. IC50 value in cell culture will provide information on the concentration at which an enzyme’s activity or receptor is 50% inhibited.
Jatrophone as new anticancer compounds much scrutinized against various human cell line due to the effect that promise as anti-cancer drugs (9), (29), (30). This study obtained the value of IC50 at 1,31 µg/ml or equivalent to 3,2051 µM. As a comparison, IC50 Jatrophone on cancer cell line WiDr is 8,97 µM, HeLa 5,13 µM (9), dan AGS 2,5 µM (30) respectively. From the results of this research showed IC50 of jatrophone compounds against cell line Hep G2 1886 is 3,2 µM. Jatrophone has better cytotoxic effects against liver cancer cell line Hep G2 1886 compared to IC50 colon cancer cell line WiDr at 8.97 µM and cervical cancer HeLa cell line at 5.13 µM. However, it has lower value when compared against the cytotoxic effect of jatrophone on cell line of gastric cancer, where on a cell line of gastric cancer IC50 jatrophone obtained amounted to 2.5 µM (30), whereas on the research on cancer hepatocelluler cell line Hep G2 obtained 3.2 µM of IC50.
This study will also be discussed comparison IC50 jatrophone when compared to the standard drugs such as doxorubicin, sorafenib and ATO (Arsenic Trioxyde). Doxorubicin has 2.2 µ M an IC50 within 24 hours of the grant on the cell Hep G2 (31), cytotoxic effect of jatrophone is lower if compared to doxorubicin. However, if compared with other standard therapies such as sorafenib and ATO is given for 24 hours on the cell Hep G2 obtained results IC50 of 9.9 µM (32) and 32.7 µM (32), then jatrophone has better anticancer activity when compared to both the drug. It can be said also hepatocellular cancer therapy using jatrophone is also very promising because jatrophone is derived from the bellyache bush, herbs grown in Indonesia that will be much inexpensive when compared to standard medication such as sorafenib and ATO.
Conclucion
Jatrophone is active compound isolated from bellyache bush that has better potential cytotoxic effect against hepatocelluler cancer cell line Hep G2 compared to colon cancer cell line WiDr and cervical cancer cell0 line HeLa. In addition jatrophone also has better anticancer effect against hepatocelluler carcinoma Hep G2 1886 compared to standard anticancer drugs such as sorafenib and ATO.
Acknowledgment
There is no conflict of interest. The authors acknowledge the entire staff of Laboratory of Research and Development of Dharmais Cancer Center Hospital Jakarta for providing research under Faculty of Medicine Lampung University 2017
Refrences
- Sjahid landyyun rahmawan. Isolasi dan identifikasi flavonoid dari daun dewandaru (Eugenia uniflora L.). 2008.
- Menkes RI. Peraturan Menteri Kesehatan Republik Indonesia No. 88 Tahun 2013. 2013;1–65.
- Asep Sukohar, Setiawan, Firman F, Wirakusumah HSS. Isolation and characterization cytotoxic comounds caffeine and chlorogenic acid seeds of lampung coffee robusta. J Med Planta. 2011;1(4):11.
- Haas W, Mittelbach M. Detoxification experiments with the seed oil from Jatropha curcas L. Ind Crops Prod. 2000;12(2):111–8.
CrossRef - Makkar HP., Becker K. Nutritional studies on rats and fish fed diets containing unheated and heated jatropha curcas. Vol. 53, Plant Foods for Human Nutrition. 1999. p. 183–92.
CrossRef - Sharma S, Dhamija HK, Parashar B. Jatropha curcas : A Review. 2012;2(3):107–11.
- Ema Sarimole. Manfaat jarak pagar (Jatropha curcas) sebagai obat tradisional. In 2014. p. 9–12.
- Félix-silva J, Giordani RB, Silva-jr AA, Zucolotto SM, Fernandes-pedrosa MDF. Jatropha gossypiifolia L. (Euphorbiaceae): A Review of traditional uses, phytochemistry, pharmacology, and toxicology of this medicinal plant Juliana. 2014;2014.
- Sahidin. Cytotoxic potency of diterpenes from jatropha plants. 2013;5(3):3–6.
- Sahidin. Terpenoids from the stem bark of jatropha plants and their biological activities. 2011;15(2):106–10.
- Theoduloz C. Antiproliferative activity of the diterpenes Jatrophone and Jatropholone and their derivatives cristina caused by Solanum chrysotrichum Saponin sc-2. Planta Med. 2009;75(14):1517–20.
CrossRef - Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-tieulent J, Jemal A. Global cancer statistics, 2012. CA a cancer J Clin [Internet]. 2015;65(2):87–108. Available from: http://onlinelibrary.wiley.com/doi/10.3322/caac.21262/abstract
CrossRef - American Cancer Society. Cancer Facts & Figures 2015. Cancer Facts Fig 2015. 2015;1–9.
- Kementrian Kesehatan RI Pusat Data dan Informasi Kesehatan. Stop kanker. infodatin-Kanker. 2015;hal 3.
- Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117(5):1019–26.
CrossRef - Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–74.
CrossRef - Leung HWC, Liu C-F, Chan ALF. Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol [Internet]. 2016;11(1):69. Available from: http://ro-journal.biomedcentral.com/articles/10.1186/s13014-016-0644-4
CrossRef - Zhang P, Yang Y, Wen F, He X, Tang R, Du Z, et al. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol [Internet]. 2015;27(7):853–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25919775
CrossRef - Permenkes no. 59. Permenkes RI No. 59 tentang standar tarif pelayanan kesehatan dalam penyelenggaraan program jaminan kesehatan. Dep Kesehat RI. 2014;7–8.
- Notas G, Kolios G, Mastrodimou N, Kampa M, Vasilaki A, Xidakis C, et al. Cortistatin production by HepG2 human hepatocellular carcinoma cell line and distribution of somatostatin receptors. J Hepatol. 2004;40(5):792–8.
CrossRef - Sabandar CW, Ahmat N, Jaafar FM, Sahidin I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry [Internet]. 2013;85:7–29. Available from: http://dx.doi.org/10.1016/j.phytochem.2012.10.009.
CrossRef - Miller LC, Tainter ML. Estimation of LD50 and its error by means of log-probit graph paper. Proc Soc Exp Bio Med 1944;57:261.
CrossRef - J. T. Litchfield, F. Wilcoxon. A simplified method of evaluating dose-effect experiments. Journal of Pharmacology and Experimental Therapeutics June 1, 1949, 96 (2) 99-113.
- Weil, C. S.: Tables for convenient calculation of median-effective dose (LD50 or ED50) and instruction in their use. Biometrics 1952, 8, 249–263.
CrossRef - Finney DJ.3rd ed. Probit analysis. Cambridge: Cambridge University Press;1971.
- Stegmeier F, Warmuth M, Sellers WR, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Ther [Internet]. 2010;87(5):543–52. Available from: http://onlinelibrary.wiley.com/store/10.1038/clpt.2009.297/asset/cptclpt2009297.pdf?v=1&t=ih3z6zh8&s=1d0612b2f4d995ce079307fe7c2db211813a5845
CrossRef - Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-based cancer therapeutics – Promise and challenge – Lessons learned through the NCI alliance for nanotechnology in cancer. Pharm Res. 2011;28(2):273–8.
CrossRef - Atkins JH, Gershell LJ. From the analyst’s couch: Selective anticancer drugs. Nat Rev Drug Discov [Internet]. 2002;1(7):491–2. Available from: http://www.nature.com/doifinder/10.1038/nrd842
CrossRef - Nakazibwe S. Study of Antiproliferation and Induction of apoptosis By Jatrophone and Curcusone B on human cancer cells. 2010;(October).
- Pertino M, Schmeda-Hirschmann G, Santos LS, Rodríguez JA, Theoduloz C. Biotransformation of jatrophone by Aspergillus niger ATCC 16404. Zeitschrift fur Naturforsch – Sect B J Chem Sci. 2007;62(2):275–9.
CrossRef - Al-Qubaisi M, Rozita R, Yeap SK, Omar AR, Ali AM, Alitheen NB. Selective cytotoxicity of goniothalamin against hepatoblastoma HepG2 cells. Molecules. 2011;16(4):2944–59.
CrossRef - Rangwala F, Williams KP, Smith GR, Thomas Z, Allensworth JL, Lyerly HK, et al. Differential effects of arsenic trioxide on chemosensitization in human hepatic tumor and stellate cell lines. BMC cancer [Internet]. 2012;12(1):402. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3517386&tool=pmcentrez&rendertype=abstract
CrossRef








