Manuscript accepted on :December 18, 2017
Published online on: --
Plagiarism Check: Yes
Silvia W. Lestari1, Manggiasih D. Larasati2, Indra G. Mansur1, Muhammad F. Soelaeman3, Favian A. Rahmat3, Fira Azzahra3 and Fariz A. Al-Rasyid3
1Department of Medical Biology, Faculty of Medicine Universitas Indonesia.
2Master Program for Biomedical Sciences, Faculty of Medicine Universitas Indonesia.
3Bachelor Program, Faculty of Medicine Universitas Indonesia.
Corresponding Author E-mail: finallysilvia@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1349
Abstract
Axoneme structures in sperm tail, is a supramolecular protein complex with motor protein and regulatory which playing a crucial role in determining sperm motility. Dynein, one of the three members of cytoskeletal motor protein, has a ring of six AAA+ which linked together into one large polypeptide that contribute to the formation of sperm flagella bending. The previously research reported that the first two AAA motor domains, AAA1 and AAA2, were a major site in ATP hydrolysis associated with motility in the flagellum. Intrauterine Insemination (IUI) as a management of infertility requires sperm preparation procedures, by Swim-up (SU) and Density Gradient Centrifugation (DGC), in order to enhance the quality regarding concentration and motility of the initial sperm. This study aimed to evaluate the efficiency of the DGC and SU methods in selecting sperm, based the expression of sperm dynein AAA1 and AAA2. Semen samples were obtained from men underwent sperm preparation for IUI and divided into two groups, normozoospermia and asthenozoospermia, according to World Health Organization 2010 guideline. Semen analysis was performed to measure the sperm motility and velocity, before and after sperm preparation. The axoneme was isolated from the obtained samples from SU and DGC methods, while the level of AAA1 and AAA2 was measured by ELISA. This study showed that the percentage of motile sperm and velocity of prepared sperm in both groups in prepared sperm (post-SU and post-DGC) was higher compared to whole semen. The expression of sperm dynein AAA1 of prepared sperm in normozoospermia group showed higher, while in asthenozoospermia group showed lower activities compared to whole semen. The expression of sperm dynein AAA2 of prepared sperm in both groups showed lower activities compared to whole semen. The sperm preparation enhanced the quality of sperm and may increase the expression of sperm dynein AAA1 compared to the whole semen, without the involvement of sperm dynein AAA2.
Keywords
AAA1;AAA2; Dynein;Density Gradient Centrifugation Intrauterine Insemination; Swim-up;
Download this article as:| Copy the following to cite this article: Lestari S. W, Larasati M. D, Mansur I. G, Soelaeman M. F, Rahmat F. A, Azzahra F, Al-Rasyid F. A. Sperm Dynein AAA1 and AAA2 Expression in Human Sperm : A Regulation in Sperm Preparation. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Lestari S. W, Larasati M. D, Mansur I. G, Soelaeman M. F, Rahmat F. A, Azzahra F, Al-Rasyid F. A. Sperm Dynein AAA1 and AAA2 Expression in Human Sperm : A Regulation in Sperm Preparation. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18921 |
Introduction
The sperm motility is determined by the axial complex in tail section called axoneme.1 Axoneme is a cytoskeleton consisting of 9 doublet of peripheral microtubules that surround 2 single of central microtubules (9 + 2 structure) and equipped with dynein arm and nexin bridge.2,3 Dynein is a complex protein composed of 1 to 3 heavy chains, together with a number of medium and light chains.4 The dynein heavy chain is involve in many motor activities such as ATP hydrolysis, ATP-sensitive microtubule binding and microtubule shift,1,5 therefore it is a fundamental motor unit.6
Dynein structure consists of motor domains of carboxy-terminal (head) and amino-terminal motor domains (tail). At the head of dynein, there are six AAA+ (ATPases associated with various cellular activities) arranged in a ring,7 of which four domains (AAA1-AAA4) contain nucleotide binder sites, while the other two domains (AAA5 and AAA6) contain less sites.5 Mutagenesis and biochemical studies suggest that AAA1 is a major site in ATP hydrolysis associated with motility in the flagellum. Recent studies suggest that hydrolysis activity in AAA1 also requires structural involvement of AAA2. In AAA2, when the binding nucleotides are removed by mutagenesis it will slow down motility by dynein.8 Therefore the first two AAA motor domains are the main energy source of dynein motors in hydrolysis of ATP.9 In other words, dynein obtained ATP from AAA domain for microtubules sliding in the axoneme that engenders sperm motility. Up till know, there are still limited data about dynein AAA protein in human sperms.
In our prime research, we analyzed about the sperm ATPase activity and the expression of sperm ATPase related protein, including Na,K-ATPase α4 and PMCA 4 isoforms in sperm motility abnormalities such as asthenozoospermia, oligoasthenoteratozoospermia (OAT) and necrozoospermia. In this study, we analyze about the regulation of sperm dynein AAA1 and AAA2 protein expression in sperm preparation. In the recent research, the highlight interest in Intrauterine Insemination (IUI), as a first line management of infertility, is the result of better sperm preparation procedures in which enhancing the quality of the sperm either concentration or motility. Current techniques such as Swim-up (SU) and the Density-gradient Centrifugation (DGC) perform by separating motile sperms from immotile sperms, leukocytes and cell debris from the seminal plasma. Nevertheless, there are still remaining issues regarding to select the better sperm preparation methods. Numerous researches have been conducted in order to clarify the appropriate technique which providing better sperm quality, which leading to higher success rates after IUI. This research was addressed to evaluate the efficiency of SU and DGC methods in selecting for sperm before being used for IUI, based the evaluation on sperm dynein AAA expression.
Materials and Methods
Semen Collection and Analysis
This study was approved by the Ethical Committee of Faculty of Medicine Universitas Indonesia. Semen samples were obtained from subjects who underwent sperm preparation for Intra Uterine Insemination (IUI), after an abstinence period of 2-3 days.10,11 Semen analysis was performed according to the guidance from World Health Organization (WHO).12 Sperm concentration and motility were done using Makler counting chamber (Sefi Medical Instruments, Haifa, Israel), whereas sperm morphology was done using Papanicolaou staining. Normozoospermia was defined if sperm concentration same as or more than 15 million/ml, sperm motility same as or more than 40% and sperm morphology same as or more than 4%. Asthenozoospermia was defined if sperm motility less than 40%.
Swim-up
SU method of sperm preparation was performed by utilizing Sperm Rinse reagent (Vitrolife, Gothenburg, Sweden). Sperm Rinse reagent and semen with 1:1 ratio were placed in a 15 ml Nunc conical tube (Thermo Scientific Nunc, New York, USA), before being centrifuged at 300xg for 10 minutes. (Thermo Scientific Centrifuge, New York, USA) Then, the obtained pellet was transferred into the tube which consisting of 1 ml of Sperm Rinse reagent. Subsequently, the tube was set at 45° in the incubator at 37° Celsius for 45 minutes. After that, sample was taken on the upper layer of the solution tenderly for further analysis.13
Density Gradient Centrifugation
DGC method of sperm preparation was performed by utilizing Sperm Grad reagent (Vitrolife, Gothenburg, Sweden). Sperm Grad reagent of 90%, 45% and semen respectively with 1:1:1 ratio were placed in a 15 ml Nunc conical tube (Thermo Scientific Nunc, New York, USA), before being centrifuged at 300xg for 20 minutes. (Thermo Scientific Centrifuge, New York, USA) Then, the obtained pellet was transferred into the tube which consisting of 1 ml of Sperm Rinse reagent. Subsequently, the tube was centrifuges again at 500xg for 10 minutes. After that, pellet was taken as sample for further analysis.13,14
Isolation of Sperm Axoneme
The obtained samples from SU and DGC method were mixed with 100 μL SDS extraction buffer (2 mL 10% SDS; 0.275 M Tris pH 6.8; 1 g sucrose, 3 ml ddH2O, protease inhibitor cocktail). Then the sample was centrifuged 8940 xg for 5 minutes. Supernatant was separated and measured for protein content by Bio-Rad micromethod of Bradford method.15 Then, this suspension was prepared for further analysis.
ELISA
Examination of AAA1 levels in semen was done by using ELISA kit sandwich (Human Dynein Heavy Chain 1, Axonemal (DNAH1) ELISA Kit MBS928073, My Bio Source, USA) which can detect AAA1 concentration at 23,5-1500 pg/ml. First, standard solutions were prepared by dilution of the stock in various concentrations (1500 pg/ml, 750 pg/ml, 375 pg/ml, 187.5 pg/ml, 94 pg/ml, 47 pg/ml and 23,5 pg/ml). The protein content of the sample has been previously equated as 15 μg in 100 μl sample. The well that plated with specific monoclonal antibody to AAA1 was then added with 100 μl standard solution and 100 μl sample. Then, the solution was inserted into each different well which then incubated at 37°C for 2 hours. After that, the solution was removed without washing it first. A 100 μl biotin antibody was added to each well, then was incubated at 37°C for 1 hour. Next, the plate was washed with buffer four times and kept for 2 minutes.16
Moreover, 100 μl of Streptavidin-Horseradish Peroxidase (HRP) was added to each well, and incubated at 37°C for 1 hour. The plate was then washed again with buffer four times and kept for 2 minutes. Then, 90 μl tetramethyl-benzidine (TMB substrate) was added to each well and incubated at 37°C for 15-30 minutes. The enzyme-substrate reaction was discontinued with the addition of 50 μl Stop Solution. The change of color was read by its absorbance with the ELISA Reader at the 450-nm wavelength. The AAA1 concentration was then determined by comparing the value of the Optical Density (OD) sample with the standard curve.16 Similar to the AAA1, the AAA2 levels were examined using the ELISA kit (Human Dynein Heavy Chain 2, Axonemal (DNAH2) ELISA Kit MBS9342693, My Bio Source, USA) which could detect AAA2 levels in the concentration range of 3.12-100 ng/ml.
Statistical Analysis
Statistical analysis was performed with SPSS 22 program. The Mann-Whitney test was implemented to analyze the study data. P<0.05 was considered to be significantly different.
Results and Discussion
Sperm Motility in Whole Semen and Prepared Sperm
The percentage of motile sperm and velocity in normozoospermia and asthenozoospermia groups were demonstrated in these following tables. (Table 1 and 2). This study showed that the percentage of motile sperm and velocity of prepared sperm in normozoospermia group in prepared sperm (post-SU and post-DGC) was higher compared to whole semen. (Table 1) Furthermore, the percentage of motile sperm in post-SU was higher compared to post-DGC. A significant level was shown in the difference of percentage of motile sperms in whole semen compared to both, in post-SU and post-DGC, whereas an insignificant level was demonstrated in the difference of velocity (p<0.05).
Table 1: The sperm motility qualities in whole semen and prepared sperm in normozoospermia group
| Whole semen | Post DGC | Post SU | p-value | |
| Percentage of | ||||
| motile sperm (%) | 62.2±4.8 | 90.5±3.7 | 96.2±4.1 | 0.017a;0.010b |
| Velocity (%) | 23.6±3.2 | 26.0±2.7 | 28.3±3.1 | 0.11a;0.230b |
Note: Values are mean ± SD; ‘a’ is the p value from the comparison of sperm from whole semen and post-DGC semen; ‘b’ is the p value from the comparison of sperm from whole semen and post-SU semen
Table 2: The sperm motility qualities in whole semen and prepared sperm in asthenozoospermia group
| Whole semen | Post DGC | Post SU | p-value | |
| Percentage of | ||||
| motile sperm (%) | 23.5±4.8 | 66.8±4.7 | 75.1±1.1 | 0.006a;0.010b |
| Velocity (%) | 20.9±4.2 | 24.2±3.7 | 25.7±1.1 | 0.070a;0.310b |
Note: Values are mean ± SD; ‘a’ is the p value from the comparison of sperm from whole semen and post-DGC semen; ‘b’ is the p value from the comparison of sperm from whole semen and post-SU semen
Similar to normozoospermia, asthenozoospermia group shown the same result as in normozoospermia group as follow the percentage of motile sperm and velocity of prepared sperm (post-SU and post-DGC) was higher compared to whole semen. (Table 2) Furthermore, the percentage of motile sperm in post-SU was higher compared to post-DGC. A significant level was shown in the difference of percentage of motile sperms in whole semen compared to both, in post-SU and post-DGC, whereas an insignificant level was demonstrated in the difference of velocity (p<0.05). This study was in agreement with Henkel et al that SU method proved to provide better result in motile sperm count compared to DGC method.13 Furthermore, this study confirmed Posada et al that higher motile sperm count in SU method increased the pregnancy rate more than DGC method.17
There were only a few studies conducted research about dynein ATPase activity in sperm. A study about the mechanism of spermicidal on human sperm based on dynein ATPase18 and another study about the addition of adenosine in dynein ATPase activity.19 This is the first study that compare the sperm dynein AAA expression in normozoospermia and asthenozoospermia samples in sperm preparation. In normozoospermia group, the expression of sperm dynein AAA1 in whole semen was shown to be 7.41 ± 1.33 ng/ml, whereas the expression of sperm dynein AAA1 in post DGC was defined to be 8.03 ± 0.45 ng/ml and post SU was defined to be 8.26 ± 0.22 ng/ml. (Fig. 1). The expression of sperm dynein AAA1 of prepared sperm in normozoospermia group showed higher activities compared to whole semen, whereas in the post SU demonstrated significant level (p=0.001).
In addition, in asthenozoospermia group, the expression of sperm dynein AAA1 was shown to be 10.90 ± 1.63 ng/ml in whole semen, whereas the expression of sperm dynein AAA1 of post DGC was defined to be 9.82 ± 0.67 ng/ml and post SU was defined to be 8.75 ± 25 ng/ml, p<0.05. (Fig. 1). The expression of sperm dynein AAA1 of prepared sperm in both, post DGC and post SU in asthenozoospermia group showed lower expression compared to whole semen significantly (p=0.001).
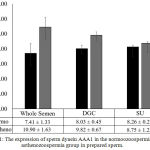 |
Figure 1: The expression of sperm dynein AAA1 in the normoozoospermia and asthenozoospermia group in prepared sperm.
|
In contrast to sperm dynein AAA1, in normozoospermia group, the expression of sperm dynein AAA2 in whole semen was shown to be 3.01 0.39 ng/ml, whereas the expression of sperm dynein AAA2 in post DGC was defined to be 2.46 0.30 ng/ml and post SU was defined to be 2.88 0.20 ng/ml. (Fig. 2). The expression of sperm dynein AAA2 of prepared sperm in normozoospermia group showed lower activities compared to whole semen, whereas in the post-DGC demonstrated insignificant level (p=0.162). In addition, in asthenozoospermia group, the expression of sperm dynein AAA2 was shown to be 2.13 0.20 ng/ml in whole semen, whereas the expression of sperm dynein AAA2 of post DGC was defined to be 0.690.35 ng/ml and post SU was defined to be 0.61 0.18 ng/ml, p<0.05. (Fig. 2). The expression of sperm dynein AAA2 of prepared sperm in both, post DGC and post SU in asthenozoospermia group showed lower expression compared to whole semen insignificantly (p=0.079; p=0.078, respectively).
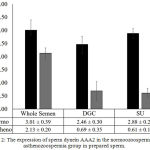 |
Figure 2: The expression of sperm dynein AAA2 in the normoozoospermia and asthenozoospermia group in prepared sperm.
|
In this study, the ELISA method measured level of expressed protein as a respond of the immune system and cell communication, therefore the obtained protein level indicate protein expression.20 Sperm dynein AAA1 demonstrated higher expression in prepared sperm compared to the whole semen.
Current techniques of sperm preparation are based on the active movement of the sperm and requirement of using repeated centrifugation to separate the sperm from the seminal plasma. The advantages that could be obtained such as gaining the clean fraction of highly motile sperm compare to the whole semen. This finding is in agreement with previous investigator, Almeida et al, that sperm preparation increased the amount of motile sperm.21,22 In our study, sperm dynein AAA1 expression was higher in post SU compared to post DGC. This finding could be due to the presence of more motile sperms that active move to the up layer at SU method.
Furthermore, this study also demonstrated a different pattern of sperm dynein AAA1 between normozoospermia and asthenozoospermia group. In normozoospermia group, the sperm dynein AAA1 expression was higher in prepared sperm compared to the whole semen. While in asthenozoospermia group, the sperm dynein AAA1 expression was lower in prepared sperm compared to the whole semen. This issue could be due to the less presence of motile sperms in asthenozoospermia which compared to normoozoospermia group.
Another finding of this study was sperm dynein AAA2 that demonstrated lower expression in prepared sperm compared to the whole semen, both in normozoospermia and asthenozoospermia group. In contrast to sperm dynein AAA1 expression, this result did not agree with the study by Almeida et al and Butt A & Chohan MA, which should actually increase the sperm dynein AAA2 expression.21,22
Even though there was study reported that sperm dynein AAA1 and AAA2 are the main energy source of dynein motors in hydrolysis ATP for microtubule sliding in sperm motility, this study re-confirmed that sperm dynein AAA1 is more fundamental compared to AAA2 in the axoneme structure.9 This result also did not agree with other previous study that AAA1 needs the role of AAA2 in sperm motility.8
In addition, the alteration in sperm protein expression after sperm preparation in this study may be possible in terms of genetic and also epigenetic. Epigenetic plays role in the regulation of male fertility, begins from spermatogenesis up to embryo development.23 Agarwal has examined alteration in the sperm protein expression in infertile men with unilateral varicoceles.24 A study from Montjean et al proved that global DNA methylation and sperm motility are interrelated.25 Moreover, Canovas has also investigated that by changing the environment of sperm or oocytes even embryos in the form of sperm preparation, the replacement of the culture medium composition with more natural fluid from reproductive system, can produce better embryos, based on DNA methylation.26 It is assumed that epigenetic modification in sperm preparation may affect the physiological or pathological conditions of sperm or semen, so it may alter the sperm protein expression.
There was a study conducted research on cytoplasmic dynein AAA3 in Saccharomyces cerevisiae as a switch of cytoplasmic dynein for cell transportation.27 Other study also reported that there was a mutation in the DNAH1 and DNAH5 in isolated asthenozoospermia.28 In addition, Neesen proved the loss of DNAH1 in infertile men.29 To the best of our knowledge, this is the first study that compare the sperm dynein AAA1 and AAA2 expression in human sperm after sperm preparation. Lastly, either structure and function damage of dynein or disruption on dynein arms may contribute to declining sperm motility and leading to male infertility.29,30
Conclusion
The sperm preparation may increase the expression of sperm dynein AAA1 compared to the whole semen, without the involvement of sperm dynein AAA2. The disruption of dynein may decline sperm motility. Further study such as epigenetic should be performed for better analysis.
Acknowledgment
The authors are grateful to Hibah PITTA DRPM 2017 for supporting this study and research assistant Meidika Dara Rizki and Debby Aditya for editorial assistance
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Source
Funding support for this study was received from Hibah Publikasi Terindeks Internasional untuk Tugas Akhir Mahasiswa (PITTA), Direktorat Riset dan Pengabdian Masyarakat (DRPM) Universitas Indonesia, 2017.
References
- Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoolog Sci. 2003;20(9):1043-56.
CrossRef - Afzelius B.A. A human syndrome caused by immotile cilia. Science. 1976;193(4250):317-9.
CrossRef - Lindemann C.B. Structural-functional relationships of the dynein, spokes, and central-pair projections predicted from an analysis of the forces acting within a flagellum. Biophys J. 2003;84(6):4115-26.
CrossRef - Porter M.E, Johnson K.A. Dynein structure and function. Annu Rev Cell Biol. 1989;5(1):119-51.
CrossRef - King S.M. The dynein microtubule motor. Biochim Biophys Acta. 2000;1496(1):60-75.
CrossRef - Nishiura M, Kon T, Shiroguchi K, Ohkura R, Shima T, Toyoshima Y.Y, et al. A single-headed recombinant fragment of Dictyostelium cytoplasmic dynein can drive the robust sliding of microtubules. J Biol Chem. 2004;279(22):22799-802.
CrossRef - Koonce M, Samso M. Overexpression of cytoplasmic dynein’s globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol Biol Cell. 1996;7(6):935-48.
CrossRef - Roberts A.J, Kon T, Knight P.J, Sutoh K, Burgess S.A. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14(11):713-26.
CrossRef - Roberts A.J, Malkova B, Walker M.L, Sakakibara H, Numata N, Kon T, et al. ATP-driven remodeling of the linker domain in the dynein motor. Structure. 2012;20(10):1670-80.
CrossRef - Jurema M.W, Vieira A.D, Bankowski B, Petrella C, Zhao Y, Wallach E, et al. Effect of ejaculatory abstinence period on the pregnancy rate after intrauterine insemination. Fertil Steril. 2005;84(3):678-81.
CrossRef - Marshburn P.B, Alanis M, Matthews M.L, Usadi R, Papadakis M.H, Kullstam S, et al. A short period of ejaculatory abstinence before intrauterine insemination is associated with higher pregnancy rates. Fertil Steril. 2010;93(1):286-8.
CrossRef - World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th Geneva: WHO Press. 2010.
- Henkel R.R, Schill W-B. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;1(1):108.
CrossRef - Bolton V, Braude P. Preparation of human spermatozoa for in vitro fertilization by isopycnic centrifugation on self-generating density gradients. Arch Androl. 1984;13(2-3):167-76.
CrossRef - Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54.
CrossRef - DNAH1 elisa kit :Human dynein, axonemal, heavy chain 1 ELISA Kit [Available from: https://www.mybiosource.com/prods/ELISA-Kit/Human/dynein-axonemal-heavy-chain-1/DNAH1/datasheet.php?products_id=928073.
- Posada M, Azuero A, Arango A, Raigosa G, Cano J, Perez A. Sperm Washing With Swim Up Versus Gradients in Intrauterine Insemination (IUI): Results of a Prospective Randomized Study Comparing Pregnancy Rates and Costs. Fertil Steril. 2005;84(1):361.
CrossRef - D’Cruz O.J, Vassilev A, Uckun F.M. Studies in humans on the mechanism of potent spermicidal and apoptosis-inducing activities of vanadocene complexes. Biol Reprod. 2000;62(4):939-49.
CrossRef - Romac P, ˇanić-Grubiśić T, Ćulić O, Cvitković P, Flogel M. Sperm motility and kinetics of dynein ATPase in astheno-and normozoospermic samples after stimulation with adenosine and its analogues. Hum Reprod. 1994;9(8):1474-8.
CrossRef - Ernawati S.R, Rahardjo A, Sianita N, Rahmahani J, Rantam F.E, dan protokol t. Surabaya: Fakultas Kedokteran Hewan, UNAIR. 2010.
- Butt F, Chohan M.A. Comparative efficacy of density gradient and swim-up methods of semen preparation in intrauterine insemination cycles. J Pak Med Assoc. 2016;66(8):932-7.
- Almeida C.F, Cardoso M, Sousa M, Viana P, Gonçalves A, Silva J, et al. Quantitative study of caspase-3 activity in semen and after swim-up preparation in relation to sperm quality. Hum Reprod. 2005;20(5):1307-13.
CrossRef - Lestari S.W, Rizki M.D. Epigenetic: A new approach to etiology of infertility. Med J Indon. 2016;25(4):255-62.
CrossRef - Agarwal A, Sharma R, Durairajanayagam D, Ayaz A, Cui Z, Willard B, et al. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13(1):8.
CrossRef - Montjean D, Zini A, Ravel C, Belloc S, Dalleac A, Copin H, et al. Sperm global DNA methylation level: association with semen parameters and genome integrity. Andrology. 2015;3(2):235-40.
CrossRef - Canovas S, Ivanova E, Romar R, García-Martínez S, Soriano-Úbeda C, García-Vázquez F.A, et al. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. eLife. 2017;6:e23670.
CrossRef - DeWitt M.A, Cypranowska C.A, Cleary F.B, Belyy V, Yildiz A. The AAA3 domain of cytoplasmic dynein acts as a switch to facilitate microtubule release. Nat Struct Mol Biol. 2015;22(1):73-80.
CrossRef - Zuccarello D, Ferlin A, Cazzadore C, Pepe A, Garolla A, Moretti A, et al. Mutations in dynein genes in patients affected by isolated non-syndromic asthenozoospermia. Hum. 2008;23(8):1957-62.
CrossRef - Neesen J, Kirschner R, Ochs M, Schmiedl A, Habermann B, Mueller C, et al. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet. 2001;10(11):1117-28.
CrossRef - Moeloek N, Ahda Y. Perbandingan kemanjuran dan keamanan beberapa preparat FSH untuk stimulasi ovarium perempuan yang mengikuti program reproduksi berbantu. Maj Kedokt Indon. 2001;51:450-4.







